Altered 8-oxoguanine glycosylase in mild cognitive impairment and late-stage Alzheimer's disease brain
- PMID: 18598755
- PMCID: PMC2745061
- DOI: 10.1016/j.freeradbiomed.2008.06.003
Altered 8-oxoguanine glycosylase in mild cognitive impairment and late-stage Alzheimer's disease brain
Abstract
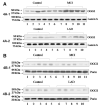
Eight-hydroxy-2'-deoxyguanosine (8-OHdG) is increased in the brain in late-stage Alzheimer's disease (LAD) and mild cognitive impairment (MCI). To determine if decreased base-excision repair contributes to these elevations, we measured oxoguanine glycosylase 1 (OGG1) protein and incision activities in nuclear and mitochondrial fractions from frontal (FL), temporal (TL), and parietal (PL) lobes from 8 MCI and 7 LAD patients, and 6 age-matched normal control (NC) subjects. OGG1 activity was significantly (P<0.05) decreased in nuclear specimens of FL, TL, and PL in MCI and LAD and in mitochondria from LAD FL and TL and MCI TL. Nuclear OGG1 protein was significantly decreased in LAD FL and MCI and LAD PL. No differences in mitochondrial OGG1 protein levels were found. Overall, our results suggest that decreased OGG1 activity occurs early in the progression of AD, possibly mediated by 4-hydroxynonenal inactivation and may contribute to elevated 8-OHdG in the brain in MCI and LAD.
Figures





Similar articles
-
Oxidatively modified nucleic acids in preclinical Alzheimer's disease (PCAD) brain.Mech Ageing Dev. 2011 Aug;132(8-9):443-8. doi: 10.1016/j.mad.2011.08.003. Epub 2011 Aug 22. Mech Ageing Dev. 2011. PMID: 21878349 Free PMC article.
-
Enzyme kinetics of an alternative splicing isoform of mitochondrial 8-oxoguanine DNA glycosylase, ogg1-1b, and compared with the nuclear ogg1-1a.J Biochem Mol Toxicol. 2015 Feb;29(2):49-56. doi: 10.1002/jbt.21605. Epub 2014 Sep 4. J Biochem Mol Toxicol. 2015. PMID: 25196052
-
Expression and polymorphisms of gene 8-oxoguanine glycosylase 1 and the level of oxidative DNA damage in peripheral blood lymphocytes of patients with Alzheimer's disease.DNA Cell Biol. 2009 Nov;28(11):579-88. doi: 10.1089/dna.2009.0926. DNA Cell Biol. 2009. PMID: 19630534
-
Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer's disease.Nucleic Acids Res. 2007;35(22):7497-504. doi: 10.1093/nar/gkm821. Epub 2007 Oct 18. Nucleic Acids Res. 2007. PMID: 17947327 Free PMC article. Review.
-
Molecular pathophysiology of impaired glucose metabolism, mitochondrial dysfunction, and oxidative DNA damage in Alzheimer's disease brain.Mech Ageing Dev. 2017 Jan;161(Pt A):95-104. doi: 10.1016/j.mad.2016.05.005. Epub 2016 May 24. Mech Ageing Dev. 2017. PMID: 27233446 Review.
Cited by
-
DNA polymerase β deficiency leads to neurodegeneration and exacerbates Alzheimer disease phenotypes.Nucleic Acids Res. 2015 Jan;43(2):943-59. doi: 10.1093/nar/gku1356. Epub 2014 Dec 30. Nucleic Acids Res. 2015. PMID: 25552414 Free PMC article.
-
Broad DNA repair responses in neural injury are associated with activation of the IL-6 pathway in cholesterol-fed rabbits.J Neurochem. 2009 Nov;111(4):1011-21. doi: 10.1111/j.1471-4159.2009.06390.x. Epub 2009 Sep 18. J Neurochem. 2009. Retraction in: J Neurochem. 2023 Aug;166(3):633. doi: 10.1111/jnc.15846. PMID: 19765189 Free PMC article. Retracted.
-
Tau Ser262 phosphorylation is critical for Abeta42-induced tau toxicity in a transgenic Drosophila model of Alzheimer's disease.Hum Mol Genet. 2010 Aug 1;19(15):2947-57. doi: 10.1093/hmg/ddq200. Epub 2010 May 12. Hum Mol Genet. 2010. PMID: 20466736 Free PMC article.
-
Adverse stress, hippocampal networks, and Alzheimer's disease.Neuromolecular Med. 2010 Mar;12(1):56-70. doi: 10.1007/s12017-009-8107-9. Epub 2009 Nov 27. Neuromolecular Med. 2010. PMID: 19943124 Free PMC article. Review.
-
DNA repair deficiency in neurodegeneration.Prog Neurobiol. 2011 Jul;94(2):166-200. doi: 10.1016/j.pneurobio.2011.04.013. Epub 2011 Apr 30. Prog Neurobiol. 2011. PMID: 21550379 Free PMC article. Review.
References
-
- Moreira PI, Honda K, Liu Q, Santos MS, Oliveira CR, Aliev G, Nunomura A, Zhu X, Smith MA, Perry G. Oxidative stress: the old enemy in Alzheimer’s disease pathophysiology. Curr. Alzheimer Res. 2005;2:403–408. - PubMed
-
- Lovell MA, Ehmann WD, Mattson MP, Markesbery WR. Elevated 4-hydroxynonenal in ventricular fluid in Alzheimer’s disease. Neurobiol. Aging. 1997;18:457–461. - PubMed
-
- Lovell MA, Xie C, Markesbery WR. Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiol. Aging. 2001;22:187–194. - PubMed
-
- Markesbery WR, Lovell MA. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer’s disease. Neurobiol. Aging. 1998;19:33–36. - PubMed
-
- Williams TI, Lynn BC, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in mild cognitive impairment and early Alzheimer’s disease. Neurobiol. Aging. 2006;27:1094–1099. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

