X-ray structure of snow flea antifreeze protein determined by racemic crystallization of synthetic protein enantiomers
- PMID: 18598029
- PMCID: PMC2719301
- DOI: 10.1021/ja8013538
X-ray structure of snow flea antifreeze protein determined by racemic crystallization of synthetic protein enantiomers
Abstract
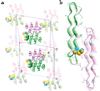
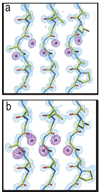
Chemical protein synthesis and racemic protein crystallization were used to determine the X-ray structure of the snow flea antifreeze protein (sfAFP). Crystal formation from a racemic solution containing equal amounts of the chemically synthesized proteins d-sfAFP and l-sfAFP occurred much more readily than for l-sfAFP alone. More facile crystal formation also occurred from a quasi-racemic mixture of d-sfAFP and l-Se-sfAFP, a chemical protein analogue that contains an additional -SeCH2- moiety at one residue and thus differs slightly from the true enantiomer. Multiple wavelength anomalous dispersion (MAD) phasing from quasi-racemate crystals was then used to determine the X-ray structure of the sfAFP protein molecule. The resulting model was used to solve by molecular replacement the X-ray structure of l-sfAFP to a resolution of 0.98 A. The l-sfAFP molecule is made up of six antiparallel left-handed PPII helixes, stacked in two sets of three, to form a compact brick-like structure with one hydrophilic face and one hydrophobic face. This is a novel experimental protein structure and closely resembles a structural model proposed for sfAFP. These results illustrate the utility of total chemical synthesis combined with racemic crystallization and X-ray crystallography for determining the unknown structure of a protein.
Figures




Similar articles
-
Mirror image forms of snow flea antifreeze protein prepared by total chemical synthesis have identical antifreeze activities.J Am Chem Soc. 2008 Jul 30;130(30):9702-7. doi: 10.1021/ja801352j. Epub 2008 Jul 4. J Am Chem Soc. 2008. PMID: 18598026 Free PMC article.
-
Single-wavelength phasing strategy for quasi-racemic protein crystal diffraction data.Acta Crystallogr D Biol Crystallogr. 2012 Jan;68(Pt 1):62-8. doi: 10.1107/S0907444911049985. Epub 2011 Dec 9. Acta Crystallogr D Biol Crystallogr. 2012. PMID: 22194334
-
Induced ice melting by the snow flea antifreeze protein from molecular dynamics simulations.J Phys Chem B. 2014 Nov 26;118(47):13527-34. doi: 10.1021/jp508992e. Epub 2014 Nov 12. J Phys Chem B. 2014. PMID: 25353109
-
Racemic & quasi-racemic protein crystallography enabled by chemical protein synthesis.Curr Opin Chem Biol. 2018 Oct;46:1-9. doi: 10.1016/j.cbpa.2018.03.012. Epub 2018 Apr 5. Curr Opin Chem Biol. 2018. PMID: 29626784 Review.
-
Recent advances in racemic protein crystallography.Bioorg Med Chem. 2017 Sep 15;25(18):4953-4965. doi: 10.1016/j.bmc.2017.05.020. Epub 2017 May 10. Bioorg Med Chem. 2017. PMID: 28705433 Review.
Cited by
-
Total chemical synthesis of human proinsulin.Chem Commun (Camb). 2010 Nov 21;46(43):8177-9. doi: 10.1039/c0cc03141k. Epub 2010 Sep 28. Chem Commun (Camb). 2010. PMID: 20877850 Free PMC article.
-
Glycine-Rich Peptides from FUS Have an Intrinsic Ability to Self-Assemble into Fibers and Networked Fibrils.Biochemistry. 2021 Nov 2;60(43):3213-3222. doi: 10.1021/acs.biochem.1c00501. Epub 2021 Oct 14. Biochemistry. 2021. PMID: 34648275 Free PMC article.
-
X-ray structure of native scorpion toxin BmBKTx1 by racemic protein crystallography using direct methods.J Am Chem Soc. 2009 Feb 4;131(4):1362-3. doi: 10.1021/ja8077973. J Am Chem Soc. 2009. PMID: 19133782 Free PMC article.
-
Quasiracemate Crystal Structures of Magainin 2 Derivatives Support the Functional Significance of the Phenylalanine Zipper Motif.J Am Chem Soc. 2015 Sep 23;137(37):11884-7. doi: 10.1021/jacs.5b07206. Epub 2015 Sep 10. J Am Chem Soc. 2015. PMID: 26369301 Free PMC article.
-
Collagen Structured Hydration.Biomolecules. 2023 Dec 4;13(12):1744. doi: 10.3390/biom13121744. Biomolecules. 2023. PMID: 38136615 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

