CD73 is required for efficient entry of lymphocytes into the central nervous system during experimental autoimmune encephalomyelitis
- PMID: 18591671
- PMCID: PMC2453691
- DOI: 10.1073/pnas.0711175105
CD73 is required for efficient entry of lymphocytes into the central nervous system during experimental autoimmune encephalomyelitis
Abstract
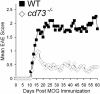
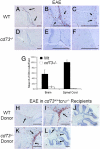
CD73 is a cell surface enzyme of the purine catabolic pathway that catalyzes the breakdown of AMP to adenosine. Because of the strong immunosuppressive and antiinflammatory properties of adenosine, we predicted that cd73(-/-) mice would develop severe experimental autoimmune encephalomyelitis (EAE), an animal model for the central nervous system (CNS) inflammatory disease, multiple sclerosis. Surprisingly, cd73(-/-) mice were resistant to EAE. However, CD4 T cells from cd73(-/-) mice secreted more proinflammatory cytokines than wild-type (WT) mice and were able to induce EAE when transferred into naïve cd73(+/+) T cell-deficient recipients. Therefore, the protection from EAE observed in cd73(-/-) mice was not caused by a deficiency in T cell responsiveness. Immunohistochemistry showed that cd73(-/-) mice had fewer infiltrating lymphocytes in their CNS compared with WT mice. Importantly, susceptibility to EAE could be induced in cd73(-/-) mice after the transfer of WT CD73(+)CD4(+) T cells, suggesting that CD73 must be expressed either on T cells or in the CNS for disease induction. In the search for the source of CD73 in the CNS that might facilitate lymphocyte migration, immunohistochemistry revealed a lack of CD73 expression on brain endothelial cells and high expression in the choroid plexus epithelium which regulates lymphocyte immunosurveillance between the blood and cerebrospinal fluid. Because blockade of adenosine receptor signaling with the A(2a) adenosine receptor-specific antagonist SCH58261 protected WT mice from EAE induction, we conclude that CD73 expression and adenosine receptor signaling are required for the efficient entry of lymphocytes into the CNS during EAE development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Chronological changes of CD4(+) and CD8(+) T cell subsets in the experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis.Tohoku J Exp Med. 2007 Dec;213(4):329-39. doi: 10.1620/tjem.213.329. Tohoku J Exp Med. 2007. PMID: 18075237
-
Host T cells are the main producers of IL-17 within the central nervous system during initiation of experimental autoimmune encephalomyelitis induced by adoptive transfer of Th1 cell lines.J Immunol. 2008 Jun 15;180(12):8066-72. doi: 10.4049/jimmunol.180.12.8066. J Immunol. 2008. PMID: 18523270
-
Extracellular adenosine signaling induces CX3CL1 expression in the brain to promote experimental autoimmune encephalomyelitis.J Neuroinflammation. 2012 Aug 10;9:193. doi: 10.1186/1742-2094-9-193. J Neuroinflammation. 2012. PMID: 22883932 Free PMC article.
-
Immune cell entry to central nervous system--current understanding and prospective therapeutic targets.Endocr Metab Immune Disord Drug Targets. 2009 Dec;9(4):315-27. doi: 10.2174/187153009789839219. Endocr Metab Immune Disord Drug Targets. 2009. PMID: 20028334 Review.
-
A2AR antagonist treatment for multiple sclerosis: Current progress and future prospects.Int Rev Neurobiol. 2023;170:185-223. doi: 10.1016/bs.irn.2023.05.012. Epub 2023 Jun 5. Int Rev Neurobiol. 2023. PMID: 37741692 Review.
Cited by
-
A2A adenosine receptor signaling in lymphocytes and the central nervous system regulates inflammation during experimental autoimmune encephalomyelitis.J Immunol. 2012 Jun 1;188(11):5713-22. doi: 10.4049/jimmunol.1200545. Epub 2012 Apr 23. J Immunol. 2012. PMID: 22529293 Free PMC article.
-
Elevated ATP via enhanced miRNA-30b, 30c, and 30e downregulates the expression of CD73 in CD8+ T cells of HIV-infected individuals.PLoS Pathog. 2022 Mar 24;18(3):e1010378. doi: 10.1371/journal.ppat.1010378. eCollection 2022 Mar. PLoS Pathog. 2022. PMID: 35325005 Free PMC article.
-
Induction of NTPDase1/CD39 by Reactive Microglia and Macrophages Is Associated With the Functional State During EAE.Front Neurosci. 2019 Apr 26;13:410. doi: 10.3389/fnins.2019.00410. eCollection 2019. Front Neurosci. 2019. PMID: 31105520 Free PMC article.
-
Loss of the tumor suppressor gene NF2, encoding merlin, constitutively activates integrin-dependent mTORC1 signaling.Mol Cell Biol. 2009 Aug;29(15):4235-49. doi: 10.1128/MCB.01578-08. Epub 2009 May 18. Mol Cell Biol. 2009. PMID: 19451229 Free PMC article.
-
CD73+ CD127high Long-Term Memory CD4 T Cells Are Highly Proliferative in Response to Recall Antigens and Are Early Targets in HIV-1 Infection.Int J Mol Sci. 2021 Jan 18;22(2):912. doi: 10.3390/ijms22020912. Int J Mol Sci. 2021. PMID: 33477692 Free PMC article.
References
-
- Keegan BM, et al. Multiple sclerosis. Annu Rev Med. 2002;53:285–302. - PubMed
-
- Niino M, et al. Recent advances in genetic analysis of multiple sclerosis: Genetic associations and therapeutic implications. Exp Rev Neurother. 2007;7:1175–1188. - PubMed
-
- Ascherio A, et al. Environmental risk factors for multiple sclerosis. II. Noninfectious factors. Ann Neurol. 2007;61:504–513. - PubMed
-
- Brown DA, et al. Time course and distribution of inflammatory and neurodegenerative events suggest structural bases for the pathogenesis of experimental autoimmune encephalomyelitis. J Comp Neurol. 2007;502:236–260. - PubMed
-
- Hemmer B, et al. Multiple sclerosis: A coordinated immune attack across the blood brain barrier. Curr Neurovasc Res. 2004;1:141–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

