Immunodominant, protective response to the parasite Toxoplasma gondii requires antigen processing in the endoplasmic reticulum
- PMID: 18587399
- PMCID: PMC4976627
- DOI: 10.1038/ni.1629
Immunodominant, protective response to the parasite Toxoplasma gondii requires antigen processing in the endoplasmic reticulum
Abstract
The parasite Toxoplasma gondii replicates in a specialized intracellular vacuole and causes disease in many species. Protection from toxoplasmosis is mediated by CD8(+) T cells, but the T. gondii antigens and host genes required for eliciting protective immunity are poorly defined. Here we identified GRA6, a polymorphic protein secreted in the parasitophorous vacuole, as the source of the immunodominant and protective decapeptide HF10 presented by the H-2L(d) major histocompatibility complex class I molecule. Presentation of the HF10-H-2L(d) ligand required proteolysis by ERAAP, the endoplasmic reticulum aminopeptidase associated with antigen processing. Consequently, expansion of protective CD8(+) T cell populations was impaired in T. gondii-infected ERAAP-deficient mice, which were more susceptible to toxoplasmosis. Thus, endoplasmic reticulum proteolysis is critical for eliciting protective immunity to a vacuolar parasite.
Figures

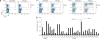
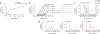
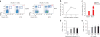

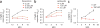
Comment in
-
Cat and mouse.Nat Immunol. 2008 Aug;9(8):829-30. doi: 10.1038/ni0808-829. Nat Immunol. 2008. PMID: 18645587 No abstract available.
Similar articles
-
Class I major histocompatibility complex presentation of antigens that escape from the parasitophorous vacuole of Toxoplasma gondii.Infect Immun. 2005 Feb;73(2):703-11. doi: 10.1128/IAI.73.2.703-711.2005. Infect Immun. 2005. PMID: 15664908 Free PMC article.
-
MHC I presentation of Toxoplasma gondii immunodominant antigen does not require Sec22b and is regulated by antigen orientation at the vacuole membrane.Eur J Immunol. 2017 Jul;47(7):1160-1170. doi: 10.1002/eji.201646859. Epub 2017 Jun 8. Eur J Immunol. 2017. PMID: 28508576
-
Transnuclear CD8 T cells specific for the immunodominant epitope Gra6 lower acute-phase Toxoplasma gondii burden.Immunology. 2016 Nov;149(3):270-279. doi: 10.1111/imm.12643. Epub 2016 Aug 17. Immunology. 2016. PMID: 27377596 Free PMC article.
-
GRA proteins of Toxoplasma gondii: maintenance of host-parasite interactions across the parasitophorous vacuolar membrane.Korean J Parasitol. 2009 Oct;47 Suppl(Suppl):S29-37. doi: 10.3347/kjp.2009.47.S.S29. Korean J Parasitol. 2009. PMID: 19885333 Free PMC article. Review.
-
Review: Toxoplasma gondii cellular invasion.Parassitologia. 1992 Dec;34(1-3):31-43. Parassitologia. 1992. PMID: 1339976 Review.
Cited by
-
NF-kappaB1 contributes to T cell-mediated control of Toxoplasma gondii in the CNS.J Neuroimmunol. 2010 May;222(1-2):19-28. doi: 10.1016/j.jneuroim.2009.12.009. Epub 2010 Feb 13. J Neuroimmunol. 2010. PMID: 20156658 Free PMC article.
-
Advances in imaging the innate and adaptive immune response to Toxoplasma gondii.Future Microbiol. 2010 Sep;5(9):1321-8. doi: 10.2217/fmb.10.97. Future Microbiol. 2010. PMID: 20860479 Free PMC article. Review.
-
Endoplasmic reticulum aminopeptidases: Biology and pathogenic potential.Nat Rev Rheumatol. 2010 Aug;6(8):461-7. doi: 10.1038/nrrheum.2010.85. Epub 2010 Jun 8. Nat Rev Rheumatol. 2010. PMID: 20531381 Review.
-
Endoplasmic reticulum aminopeptidase associated with antigen processing defines the composition and structure of MHC class I peptide repertoire in normal and virus-infected cells.J Immunol. 2010 Mar 15;184(6):3033-42. doi: 10.4049/jimmunol.0903712. Epub 2010 Feb 19. J Immunol. 2010. PMID: 20173027 Free PMC article.
-
Complex immune cell interplay in the gamma interferon response during Toxoplasma gondii infection.Infect Immun. 2014 Aug;82(8):3090-7. doi: 10.1128/IAI.01722-14. Epub 2014 May 27. Infect Immun. 2014. PMID: 24866795 Free PMC article. Review.
References
-
- Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. - PubMed
-
- Yap GS, Sher A. Cell-mediated immunity to Toxoplasma gondii: initiation, regulation and effector function. Immunobiology. 1999;201:240–247. - PubMed
-
- Gazzinelli RT, Eltoum I, Wynn TA, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-α and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993;151:3672–3681. - PubMed
-
- Schluter D, et al. Both lymphotoxin-α and TNF are crucial for control of Toxoplasma gondii in the central nervous system. J Immunol. 2003;170:6172–6182. - PubMed
-
- Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

