Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes
- PMID: 18586852
- PMCID: PMC2547444
- DOI: 10.1529/biophysj.107.124545
Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes
Abstract
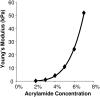
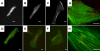
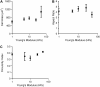
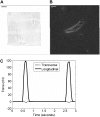
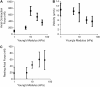
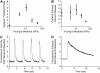
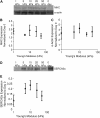
Cardiac cells mature in the first postnatal week, concurrent with altered extracellular mechanical properties. To investigate the effects of extracellular stiffness on cardiomyocyte maturation, we plated neonatal rat ventricular myocytes for 7 days on collagen-coated polyacrylamide gels with varying elastic moduli. Cells on 10 kPa substrates developed aligned sarcomeres, whereas cells on stiffer substrates had unaligned sarcomeres and stress fibers, which are not observed in vivo. We found that cells generated greater mechanical force on gels with stiffness similar to the native myocardium, 10 kPa, than on stiffer or softer substrates. Cardiomyocytes on 10 kPa gels also had the largest calcium transients, sarcoplasmic calcium stores, and sarcoplasmic/endoplasmic reticular calcium ATPase2a expression, but no difference in contractile protein. We hypothesized that inhibition of stress fiber formation might allow myocyte maturation on stiffer substrates. Treatment of maturing cardiomyocytes with hydroxyfasudil, an inhibitor of RhoA kinase and stress fiber-formation, resulted in enhanced force generation on the stiffest gels. We conclude that extracellular stiffness near that of native myocardium significantly enhances neonatal rat ventricular myocytes maturation. Deviations from ideal stiffness result in lower expression of sarcoplasmic/endoplasmic reticular calcium ATPase, less stored calcium, smaller calcium transients, and lower force. On very stiff substrates, this adaptation seems to involve RhoA kinase.
Figures
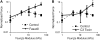
Similar articles
-
Cardiac myocyte force development during differentiation and maturation.Ann N Y Acad Sci. 2010 Feb;1188:121-7. doi: 10.1111/j.1749-6632.2009.05091.x. Ann N Y Acad Sci. 2010. PMID: 20201894 Free PMC article.
-
Reprogramming cardiomyocyte mechanosensing by crosstalk between integrins and hyaluronic acid receptors.J Biomech. 2012 Mar 15;45(5):824-31. doi: 10.1016/j.jbiomech.2011.11.023. Epub 2011 Dec 24. J Biomech. 2012. PMID: 22196970 Free PMC article.
-
The Effect of Substrate Stiffness on Cardiomyocyte Action Potentials.Cell Biochem Biophys. 2016 Dec;74(4):527-535. doi: 10.1007/s12013-016-0758-1. Epub 2016 Oct 8. Cell Biochem Biophys. 2016. PMID: 27722948 Free PMC article.
-
Rho-kinase inhibition reverses impaired Ca2+ handling and associated left ventricular dysfunction in pressure overload-induced cardiac hypertrophy.Cell Calcium. 2017 Nov;67:81-90. doi: 10.1016/j.ceca.2017.09.002. Epub 2017 Sep 9. Cell Calcium. 2017. PMID: 29029794
-
Effect of substrate mechanics on cardiomyocyte maturation and growth.Tissue Eng Part B Rev. 2015 Feb;21(1):157-65. doi: 10.1089/ten.TEB.2014.0383. Epub 2014 Nov 12. Tissue Eng Part B Rev. 2015. PMID: 25148904 Free PMC article. Review.
Cited by
-
A role for matrix stiffness in the regulation of cardiac side population cell function.Am J Physiol Heart Circ Physiol. 2015 May 1;308(9):H990-7. doi: 10.1152/ajpheart.00935.2014. Epub 2015 Feb 27. Am J Physiol Heart Circ Physiol. 2015. PMID: 25724498 Free PMC article.
-
Hydrogel scaffolds with elasticity-mimicking embryonic substrates promote cardiac cellular network formation.Prog Biomater. 2020 Sep;9(3):125-137. doi: 10.1007/s40204-020-00137-0. Epub 2020 Sep 25. Prog Biomater. 2020. PMID: 32978746 Free PMC article.
-
Cardiac fibroblasts and mechanosensation in heart development, health and disease.Nat Rev Cardiol. 2023 May;20(5):309-324. doi: 10.1038/s41569-022-00799-2. Epub 2022 Nov 14. Nat Rev Cardiol. 2023. PMID: 36376437 Review.
-
Hydrogel crosslinking density regulates temporal contractility of human embryonic stem cell-derived cardiomyocytes in 3D cultures.Soft Matter. 2012 Oct 21;8(39):10141-10148. doi: 10.1039/C2SM26082D. Epub 2012 Aug 21. Soft Matter. 2012. PMID: 23226161 Free PMC article.
-
Electrical and mechanical stimulation of cardiac cells and tissue constructs.Adv Drug Deliv Rev. 2016 Jan 15;96:135-55. doi: 10.1016/j.addr.2015.07.009. Epub 2015 Jul 30. Adv Drug Deliv Rev. 2016. PMID: 26232525 Free PMC article. Review.
References
-
- Hudlicka, O., and M. D. Brown. 1996. Postnatal growth of the heart and its blood vessels. J. Vasc. Res. 33:266–287. - PubMed
-
- Fukuda, K., and S. Yuasa. 2006. Stem cells as a source of regenerative cardiomyocytes. Circ. Res. 98:1002–1013. - PubMed
-
- Dowell, J. D., M. Rubart, K. B. Pasumarthi, M. H. Soonpaa, and L. J. Field. 2003. Myocyte and myogenic stem cell transplantation in the heart. Cardiovasc. Res. 58:336–350. - PubMed
-
- Menasche, P. 2005. The potential of embryonic stem cells to treat heart disease. Curr. Opin. Mol. Ther. 7:293–299. - PubMed
-
- Srivastava, D., and K. N. Ivey. 2006. Potential of stem-cell-based therapies for heart disease. Nature. 441:1097–1099. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

