Toll-like receptor-4 mediates neuronal apoptosis induced by amyloid beta-peptide and the membrane lipid peroxidation product 4-hydroxynonenal
- PMID: 18586243
- PMCID: PMC2597513
- DOI: 10.1016/j.expneurol.2008.05.014
Toll-like receptor-4 mediates neuronal apoptosis induced by amyloid beta-peptide and the membrane lipid peroxidation product 4-hydroxynonenal
Abstract
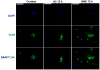
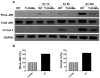
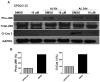
The innate immune system senses the invasion of pathogenic microorganisms and tissue injury through Toll-like receptors (TLR), a mechanism thought to be limited to immune cells. We recently found that neurons express several TLRs, and that the levels of TLR2 and TLR4 are increased in neurons in response to energy deprivation. Here we report that TLR4 expression increases in neurons when exposed to amyloid beta-peptide (Abeta1-42) or the lipid peroxidation product 4-hydroxynonenal (HNE). Neuronal apoptosis triggered by Abeta and HNE was mediated by jun N-terminal kinase (JNK); neurons from TLR4 mutant mice exhibited reduced JNK and caspase-3 activation and were protected against apoptosis induced by Abeta and HNE. Levels of TLR4 were decreased in inferior parietal cortex tissue specimens from end-stage AD patients compared to aged-matched control subjects, possibly as the result of loss of neurons expressing TLR4. Our findings suggest that TLR4 signaling increases the vulnerability of neurons to Abeta and oxidative stress in AD, and identify TLR4 as a potential therapeutic target for AD.
Figures

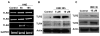





Similar articles
-
H2O2 and 4-hydroxynonenal mediate amyloid beta-induced neuronal apoptosis by activating JNKs and p38MAPK.Exp Neurol. 2003 Apr;180(2):144-55. doi: 10.1016/s0014-4886(02)00059-6. Exp Neurol. 2003. PMID: 12684028
-
Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer's disease brain contribute to neuronal death.Neurobiol Aging. 2002 Sep-Oct;23(5):655-64. doi: 10.1016/s0197-4580(01)00340-2. Neurobiol Aging. 2002. PMID: 12392766 Review.
-
Pinocembrin protects against β-amyloid-induced toxicity in neurons through inhibiting receptor for advanced glycation end products (RAGE)-independent signaling pathways and regulating mitochondrion-mediated apoptosis.BMC Med. 2012 Sep 18;10:105. doi: 10.1186/1741-7015-10-105. BMC Med. 2012. PMID: 22989295 Free PMC article.
-
Chitosan oligosaccharides protect rat primary hippocampal neurons from oligomeric β-amyloid 1-42-induced neurotoxicity.Neurosci Lett. 2013 Oct 25;554:64-9. doi: 10.1016/j.neulet.2013.08.046. Epub 2013 Aug 30. Neurosci Lett. 2013. PMID: 23999027
-
Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer's disease brain. A review.Free Radic Res. 2002 Dec;36(12):1307-13. doi: 10.1080/1071576021000049890. Free Radic Res. 2002. PMID: 12607822 Review.
Cited by
-
Glaucomatous tissue stress and the regulation of immune response through glial Toll-like receptor signaling.Invest Ophthalmol Vis Sci. 2010 Nov;51(11):5697-707. doi: 10.1167/iovs.10-5407. Epub 2010 Jun 10. Invest Ophthalmol Vis Sci. 2010. PMID: 20538986 Free PMC article.
-
Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development.World J Gastroenterol. 2010 Mar 21;16(11):1304-13. doi: 10.3748/wjg.v16.i11.1304. World J Gastroenterol. 2010. PMID: 20238396 Free PMC article. Review.
-
Atorvastatin Attenuates Cognitive Deficits and Neuroinflammation Induced by Aβ1-42 Involving Modulation of TLR4/TRAF6/NF-κB Pathway.J Mol Neurosci. 2018 Mar;64(3):363-373. doi: 10.1007/s12031-018-1032-3. Epub 2018 Feb 7. J Mol Neurosci. 2018. PMID: 29417448
-
Intermittent fasting attenuates lipopolysaccharide-induced neuroinflammation and memory impairment.J Neuroinflammation. 2014 May 6;11:85. doi: 10.1186/1742-2094-11-85. J Neuroinflammation. 2014. PMID: 24886300 Free PMC article.
-
Toll-Like Receptor 4 Deficiency Impairs Motor Coordination.Front Neurosci. 2016 Feb 16;10:33. doi: 10.3389/fnins.2016.00033. eCollection 2016. Front Neurosci. 2016. PMID: 26909014 Free PMC article.
References
-
- Al Qteishat A, Gaffney JJ, Krupinski J, Slevin M. Hyaluronan expression following middle cerebral artery occlusion in the rat. Neuroreport. 2006;17:1111–1114. - PubMed
-
- Al'Qteishat A, Gaffney J, Krupinski J, Rubio F, West D, Kumar S, Kumar P, Mitsios N, Slevin M. Changes in hyaluronan production and metabolism following ischaemic stroke in man. Brain. 2006;129:2158–2176. - PubMed
-
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. - PubMed
-
- Barton GM. Viral recognition by Toll-like receptors. Semin Immunol. 2007;19:33–40. - PubMed
-
- Beutler B. The Toll-like receptors: analysis by forward genetic methods. Immunogenetics. 2005;57:385–392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

