Temporal requirements of the fragile X mental retardation protein in the regulation of synaptic structure
- PMID: 18579676
- PMCID: PMC2753511
- DOI: 10.1242/dev.022244
Temporal requirements of the fragile X mental retardation protein in the regulation of synaptic structure
Abstract
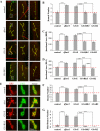
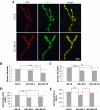
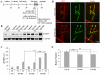
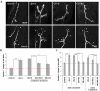
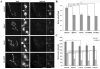
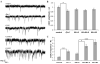
Fragile X syndrome (FraX), caused by the loss-of-function of one gene (FMR1), is the most common inherited form of both mental retardation and autism spectrum disorders. The FMR1 product (FMRP) is an mRNA-binding translation regulator that mediates activity-dependent control of synaptic structure and function. To develop any FraX intervention strategy, it is essential to define when and where FMRP loss causes the manifestation of synaptic defects, and whether the reintroduction of FMRP can restore normal synapse properties. In the Drosophila FraX model, dFMRP loss causes neuromuscular junction (NMJ) synapse over-elaboration (overgrowth, overbranching, excess synaptic boutons), accumulation of development-arrested satellite boutons, and altered neurotransmission. We used the Gene-Switch method to conditionally drive dFMRP expression to define the spatiotemporal requirements in synaptic mechanisms. Constitutive induction of targeted neuronal dFMRP at wild-type levels rescues all synaptic architectural defects in Drosophila Fmr1 (dfmr1)-null mutants, demonstrating a presynaptic requirement for synapse structuring. By contrast, presynaptic dFMRP expression does not ameliorate functional neurotransmission defects, indicating a postsynaptic dFMRP requirement. Strikingly, targeted early induction of dFMRP effects nearly complete rescue of synaptic structure defects, showing a primarily early-development role. In addition, acute dFMRP expression at maturity partially alleviates dfmr1-null defects, although rescue is not as complete as either early or constitutive dFMRP expression, showing a modest capacity for late-stage structural plasticity. We conclude that dFMRP predominantly acts early in synaptogenesis to modulate architecture, but that late dFMRP introduction at maturity can weakly compensate for early absence of dFMRP function.
Figures


Similar articles
-
The Drosophila fragile X gene negatively regulates neuronal elaboration and synaptic differentiation.Curr Biol. 2004 Oct 26;14(20):1863-70. doi: 10.1016/j.cub.2004.09.085. Curr Biol. 2004. PMID: 15498496
-
In vivo neuronal function of the fragile X mental retardation protein is regulated by phosphorylation.Hum Mol Genet. 2012 Feb 15;21(4):900-15. doi: 10.1093/hmg/ddr527. Epub 2011 Nov 11. Hum Mol Genet. 2012. PMID: 22080836 Free PMC article.
-
Fragile X mental retardation protein has a unique, evolutionarily conserved neuronal function not shared with FXR1P or FXR2P.Dis Model Mech. 2010 Jul-Aug;3(7-8):471-85. doi: 10.1242/dmm.004598. Epub 2010 May 4. Dis Model Mech. 2010. PMID: 20442204 Free PMC article.
-
Fragile X Mental Retardation Protein Regulates Activity-Dependent Membrane Trafficking and Trans-Synaptic Signaling Mediating Synaptic Remodeling.Front Mol Neurosci. 2018 Jan 12;10:440. doi: 10.3389/fnmol.2017.00440. eCollection 2017. Front Mol Neurosci. 2018. PMID: 29375303 Free PMC article. Review.
-
dFmr1 Plays Roles in Small RNA Pathways of Drosophila melanogaster.Int J Mol Sci. 2017 May 16;18(5):1066. doi: 10.3390/ijms18051066. Int J Mol Sci. 2017. PMID: 28509881 Free PMC article. Review.
Cited by
-
Increasing our understanding of human cognition through the study of Fragile X Syndrome.Dev Neurobiol. 2014 Feb;74(2):147-77. doi: 10.1002/dneu.22096. Epub 2013 Jul 30. Dev Neurobiol. 2014. PMID: 23723176 Free PMC article. Review.
-
Independent role for presynaptic FMRP revealed by an FMR1 missense mutation associated with intellectual disability and seizures.Proc Natl Acad Sci U S A. 2015 Jan 27;112(4):949-56. doi: 10.1073/pnas.1423094112. Epub 2015 Jan 5. Proc Natl Acad Sci U S A. 2015. PMID: 25561520 Free PMC article.
-
The fragile X mental retardation protein in circadian rhythmicity and memory consolidation.Mol Neurobiol. 2009 Apr;39(2):107-29. doi: 10.1007/s12035-009-8057-0. Epub 2009 Feb 12. Mol Neurobiol. 2009. PMID: 19214804 Free PMC article. Review.
-
Temporal control of a dendritogenesis-linked gene via REST-dependent regulation of nuclear factor I occupancy.Mol Biol Cell. 2011 Mar 15;22(6):868-79. doi: 10.1091/mbc.E10-10-0817. Epub 2011 Jan 26. Mol Biol Cell. 2011. PMID: 21270437 Free PMC article.
-
Modeling Fragile X Syndrome in Drosophila.Front Mol Neurosci. 2018 Apr 16;11:124. doi: 10.3389/fnmol.2018.00124. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29713264 Free PMC article. Review.
References
-
- Bailey DB, Jr., Hatton DD, Skinner M, Mesibov G. Autistic behavior, FMR1 protein, and developmental trajectories in young males with fragile X syndrome. J Autism Dev Disord. 2001a;31:165–74. - PubMed
-
- Bailey DB, Jr., Hatton DD, Tassone F, Skinner M, Taylor AK. Variability in FMRP and early development in males with fragile X syndrome. Am J Ment Retard. 2001b;106:16–27. - PubMed
-
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

