CD81 is a central regulator of cellular events required for hepatitis C virus infection of human hepatocytes
- PMID: 18579606
- PMCID: PMC2519688
- DOI: 10.1128/JVI.00665-08
CD81 is a central regulator of cellular events required for hepatitis C virus infection of human hepatocytes
Abstract
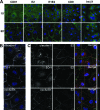
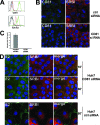
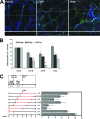
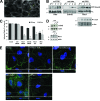
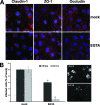
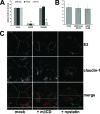
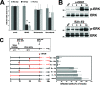
Infection with hepatitis C virus (HCV) is still a major public health problem, and the events leading to hepatocyte infection are not yet fully understood. Combining confocal microscopy with biochemical analysis and studies of infection requirements using pharmacological inhibitors and small interfering RNAs, we show here that engagement of CD81 activates the Rho GTPase family members Rac, Rho, and Cdc42 and that the block of these signaling pathways drastically reduces HCV infectivity. Activation of Rho GTPases mediates actin-dependent relocalization of the HCV E2/CD81 complex to cell-cell contact areas where CD81 comes into contact with the tight-junction proteins occludin, ZO-1, and claudin-1, which was recently described as an HCV coreceptor. Finally, we show that CD81 engagement activates the Raf/MEK/ERK signaling cascade and that this pathway affects postentry events of the virus life cycle. In conclusion, we describe a range of cellular events that are manipulated by HCV to coordinate interactions with its multiple coreceptors and to establish productive infections and find that CD81 is a central regulator of these events.
Figures
Similar articles
-
Diverse hepatitis C virus glycoproteins mediate viral infection in a CD81-dependent manner.J Virol. 2004 Aug;78(16):8496-505. doi: 10.1128/JVI.78.16.8496-8505.2004. J Virol. 2004. PMID: 15280458 Free PMC article.
-
CD81 and claudin 1 coreceptor association: role in hepatitis C virus entry.J Virol. 2008 May;82(10):5007-20. doi: 10.1128/JVI.02286-07. Epub 2008 Mar 12. J Virol. 2008. PMID: 18337570 Free PMC article.
-
CD81 is required for hepatitis C virus glycoprotein-mediated viral infection.J Virol. 2004 Feb;78(3):1448-55. doi: 10.1128/jvi.78.3.1448-1455.2004. J Virol. 2004. PMID: 14722300 Free PMC article.
-
Hepatitis C virus entry and the tetraspanin CD81.Biochem Soc Trans. 2011 Apr;39(2):532-6. doi: 10.1042/BST0390532. Biochem Soc Trans. 2011. PMID: 21428934 Review.
-
The hepatitis C virus and its hepatic environment: a toxic but finely tuned partnership.Biochem J. 2009 Oct 12;423(3):303-14. doi: 10.1042/BJ20091000. Biochem J. 2009. PMID: 19807698 Review.
Cited by
-
Strategies for targeting tetraspanin proteins: potential therapeutic applications in microbial infections.BioDrugs. 2009;23(6):341-59. doi: 10.2165/11315650-000000000-00000. BioDrugs. 2009. PMID: 19894777 Free PMC article. Review.
-
A role for CD81 and hepatitis C virus in hepatoma mobility.Viruses. 2014 Mar 24;6(3):1454-72. doi: 10.3390/v6031454. Viruses. 2014. PMID: 24662676 Free PMC article.
-
Rho'ing in and out of cells: viral interactions with Rho GTPase signaling.Small GTPases. 2014;5:e28318. doi: 10.4161/sgtp.28318. Epub 2014 Mar 24. Small GTPases. 2014. PMID: 24691164 Free PMC article. Review.
-
How hepatitis C virus invades hepatocytes: the mystery of viral entry.World J Gastroenterol. 2014 Apr 7;20(13):3457-67. doi: 10.3748/wjg.v20.i13.3457. World J Gastroenterol. 2014. PMID: 24707128 Free PMC article. Review.
-
Integrative functional genomics of hepatitis C virus infection identifies host dependencies in complete viral replication cycle.PLoS Pathog. 2014 May 22;10(5):e1004163. doi: 10.1371/journal.ppat.1004163. eCollection 2014 May. PLoS Pathog. 2014. PMID: 24852294 Free PMC article.
References
-
- Bartosch, B., A. Vitelli, C. Granier, C. Goujon, J. Dubuisson, S. Pascale, E. Scarselli, R. Cortese, A. Nicosia, and F. L. Cosset. 2003. Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J. Biol. Chem. 27841624-41630. - PubMed
-
- Berditchevski, F. 2001. Complexes of tetraspanins with integrins: more than meets the eye. J. Cell Sci. 1144143-4151. - PubMed
-
- Bernards, A., and J. Settleman. 2004. GAP control: regulating the regulators of small GTPases. Trends Cell Biol. 14377-385. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

