Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets
- PMID: 18573906
- PMCID: PMC2442646
- DOI: 10.1084/jem.20071681
Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets
Abstract
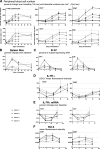
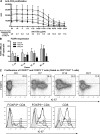
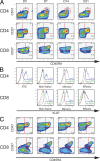
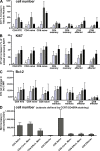
Interleukin-7 (IL-7) is a homeostatic cytokine for resting T cells with increasing serum and tissue levels during T cell depletion. In preclinical studies, IL-7 therapy exerts marked stimulating effects on T cell immune reconstitution in mice and primates. First-in-human clinical studies of recombinant human IL-7 (rhIL-7) provided the opportunity to investigate the effects of IL-7 therapy on lymphocytes in vivo. rhIL-7 induced in vivo T cell cycling, bcl-2 up-regulation, and a sustained increase in peripheral blood CD4(+) and CD8(+) T cells. This T cell expansion caused a significant broadening of circulating T cell receptor (TCR) repertoire diversity independent of the subjects' age as naive T cells, including recent thymic emigrants (RTEs), expanded preferentially, whereas the proportions of regulatory T (T reg) cells and senescent CD8(+) effectors diminished. The resulting composition of the circulating T cell pool more closely resembled that seen earlier in life. This profile, distinctive among cytokines under clinical development, suggests that rhIL-7 therapy could enhance and broaden immune responses, particularly in individuals with limited naive T cells and diminished TCR repertoire diversity, as occurs after physiological (age), pathological (human immunodeficiency virus), or iatrogenic (chemotherapy) lymphocyte depletion.
Figures



Similar articles
-
Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study.Clin Infect Dis. 2012 Jul;55(2):291-300. doi: 10.1093/cid/cis383. Epub 2012 May 1. Clin Infect Dis. 2012. PMID: 22550117 Free PMC article. Clinical Trial.
-
IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection.Blood. 2009 Jun 18;113(25):6304-14. doi: 10.1182/blood-2008-10-186601. Epub 2009 Apr 20. Blood. 2009. PMID: 19380868 Free PMC article. Clinical Trial.
-
Treatment with Interleukin-7 Restores Host Defense against Pneumocystis in CD4+ T-Lymphocyte-Depleted Mice.Infect Immun. 2015 Oct 19;84(1):108-19. doi: 10.1128/IAI.01189-15. Print 2016 Jan. Infect Immun. 2015. PMID: 26483405 Free PMC article.
-
Cytokine synergy in antigen-independent activation and priming of naive CD8+ T lymphocytes.Crit Rev Immunol. 2009;29(3):219-39. doi: 10.1615/critrevimmunol.v29.i3.30. Crit Rev Immunol. 2009. PMID: 19538136 Review.
-
Thymic function in HIV-infection.Dan Med J. 2013 Apr;60(4):B4622. Dan Med J. 2013. PMID: 23651726 Review.
Cited by
-
Incomplete immune recovery in HIV infection: mechanisms, relevance for clinical care, and possible solutions.Clin Dev Immunol. 2012;2012:670957. doi: 10.1155/2012/670957. Epub 2012 Mar 14. Clin Dev Immunol. 2012. PMID: 22474480 Free PMC article. Review.
-
Improving immunity in the elderly: current and future lessons from nonhuman primate models.Age (Dordr). 2012 Oct;34(5):1157-68. doi: 10.1007/s11357-011-9353-y. Epub 2011 Dec 20. Age (Dordr). 2012. PMID: 22180097 Free PMC article. Review.
-
Intrathymic IL-7: the where, when, and why of IL-7 signaling during T cell development.Semin Immunol. 2012 Jun;24(3):151-8. doi: 10.1016/j.smim.2012.02.002. Epub 2012 Mar 14. Semin Immunol. 2012. PMID: 22421571 Free PMC article. Review.
-
Occurrence of Anti-Drug Antibodies against Interferon-Beta and Natalizumab in Multiple Sclerosis: A Collaborative Cohort Analysis.PLoS One. 2016 Nov 2;11(11):e0162752. doi: 10.1371/journal.pone.0162752. eCollection 2016. PLoS One. 2016. PMID: 27806057 Free PMC article.
-
IL-7 and SCF Levels Inversely Correlate with T Cell Reconstitution and Clinical Outcomes after Cord Blood Transplantation in Adults.PLoS One. 2015 Jul 15;10(7):e0132564. doi: 10.1371/journal.pone.0132564. eCollection 2015. PLoS One. 2015. PMID: 26177551 Free PMC article.
References
-
- Blattman, J.N., J.M. Grayson, E.J. Wherry, S.M. Kaech, K.A. Smith, and R. Ahmed. 2003. Therapeutic use of IL-2 to enhance antiviral T cell responses in vivo. Nat. Med. 9:540–547. - PubMed
-
- Khan, I.A., and L. Casciotti. 1999. IL-15 prolongs the duration of CD8+ T cell-mediated immunity in mice infected with a vaccine strain of Toxoplasma gondii. J. Immunol. 163:4503–4509. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

