JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy
- PMID: 18570871
- PMCID: PMC2478643
- DOI: 10.1016/j.molcel.2008.06.001
JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy
Abstract
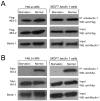
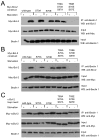
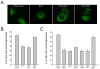
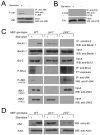
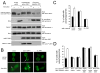
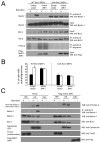
Starvation induces autophagy to preserve cellular homeostasis in virtually all eukaryotic organisms. However, the mechanisms by which starvation induces autophagy are not completely understood. In mammalian cells, the antiapoptotic protein, Bcl-2, binds to Beclin 1 during nonstarvation conditions and inhibits its autophagy function. Here we show that starvation induces phosphorylation of cellular Bcl-2 at residues T69, S70, and S87 of the nonstructured loop; Bcl-2 dissociation from Beclin 1; and autophagy activation. In contrast, viral Bcl-2, which lacks the phosphorylation site-containing nonstructured loop, fails to dissociate from Beclin 1 during starvation. Furthermore, the stress-activated signaling molecule, c-Jun N-terminal protein kinase 1 (JNK1), but not JNK2, mediates starvation-induced Bcl-2 phosphorylation, Bcl-2 dissociation from Beclin 1, and autophagy activation. Together, our findings demonstrate that JNK1-mediated multisite phosphorylation of Bcl-2 stimulates starvation-induced autophagy by disrupting the Bcl-2/Beclin 1 complex. These findings define a mechanism that cells use to regulate autophagic activity in response to nutrient status.
Figures

Similar articles
-
Dual role of JNK1-mediated phosphorylation of Bcl-2 in autophagy and apoptosis regulation.Autophagy. 2008 Oct;4(7):949-51. doi: 10.4161/auto.6788. Epub 2008 Oct 14. Autophagy. 2008. PMID: 18769111 Free PMC article.
-
The GST-BHMT assay reveals a distinct mechanism underlying proteasome inhibition-induced macroautophagy in mammalian cells.Autophagy. 2015;11(5):812-32. doi: 10.1080/15548627.2015.1034402. Autophagy. 2015. PMID: 25984893 Free PMC article.
-
Expression of cFLIPL Determines the Basal Interaction of Bcl-2 With Beclin-1 and Regulates p53 Dependent Ubiquitination of Beclin-1 During Autophagic Stress.J Cell Biochem. 2016 Aug;117(8):1757-68. doi: 10.1002/jcb.25474. Epub 2016 Feb 2. J Cell Biochem. 2016. PMID: 26682748
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
-
The Beclin 1 network regulates autophagy and apoptosis.Cell Death Differ. 2011 Apr;18(4):571-80. doi: 10.1038/cdd.2010.191. Epub 2011 Feb 11. Cell Death Differ. 2011. PMID: 21311563 Free PMC article. Review.
Cited by
-
Macrophage Perspectives in Liver Diseases: Programmed Death, Related Biomarkers, and Targeted Therapy.Biomolecules. 2024 Jun 14;14(6):700. doi: 10.3390/biom14060700. Biomolecules. 2024. PMID: 38927103 Free PMC article. Review.
-
Autophagy: New Questions from Recent Answers.ISRN Mol Biol. 2012 Dec 30;2012:738718. doi: 10.5402/2012/738718. eCollection 2012. ISRN Mol Biol. 2012. PMID: 27335669 Free PMC article. Review.
-
Modulation of apoptosis pathways by oxidative stress and autophagy in β cells.Exp Diabetes Res. 2012;2012:647914. doi: 10.1155/2012/647914. Epub 2012 Mar 12. Exp Diabetes Res. 2012. PMID: 22474427 Free PMC article.
-
Functions of BCL-X L at the Interface between Cell Death and Metabolism.Int J Cell Biol. 2013;2013:705294. doi: 10.1155/2013/705294. Epub 2013 Feb 28. Int J Cell Biol. 2013. PMID: 23533418 Free PMC article.
-
Co-Targeting of JNK and HUNK in Resistant HER2-Positive Breast Cancer.PLoS One. 2016 Apr 5;11(4):e0153025. doi: 10.1371/journal.pone.0153025. eCollection 2016. PLoS One. 2016. PMID: 27045589 Free PMC article.
References
-
- Blagosklonny MV. Unwinding the loop of Bcl-2 phosphorylation. Leukemia. 2001;15:869–874. - PubMed
-
- Bogoyevitch MA. The isoform-specific functions of the c-Jn N-terminal Kinases (JNKs): differences revealed by gene targeting. BioEssays. 2006;28:923–934. - PubMed
-
- Borsello T, Croquelois K, Hornung JP, Clarke PG. N-methyl-D-aspartate-triggered neuronal death in organotypic hippocampal clutures is endocytic, autophagic and mediated by the c-Jun N-terminal kinase pathway. Eur J Neurosci. 2003;18:473–485. - PubMed
-
- Danial NN, Korsmeyer SJ. Cell death: Critical control points. Cell. 2004;116:205–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AI051367/AI/NIAID NIH HHS/United States
- R01 CA084254/CA/NCI NIH HHS/United States
- R21 AI078108/AI/NIAID NIH HHS/United States
- R01 CA084254-09/CA/NCI NIH HHS/United States
- R01 CA109618-04/CA/NCI NIH HHS/United States
- R01AI051367/AI/NIAID NIH HHS/United States
- R01 AI051367/AI/NIAID NIH HHS/United States
- R01 CA109618/CA/NCI NIH HHS/United States
- R01CA084254/CA/NCI NIH HHS/United States
- R01CA108618/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 AI051367-06/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

