Two viral kinases are required for sustained long distance axon transport of a neuroinvasive herpesvirus
- PMID: 18564370
- PMCID: PMC3746517
- DOI: 10.1111/j.1600-0854.2008.00782.x
Two viral kinases are required for sustained long distance axon transport of a neuroinvasive herpesvirus
Abstract
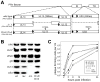
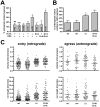
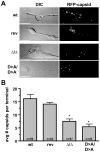
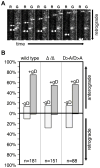
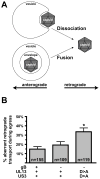
Axonal transport is essential for the successful establishment of neuroinvasive herpesvirus infections in peripheral ganglia (retrograde transport) and the subsequent spread to exposed body surfaces following reactivation from latency (anterograde transport). We examined two components of pseudorabies virus (US3 and UL13), both of which are protein kinases, as potential regulators of axon transport. Following replication of mutant viruses lacking kinase activity, newly assembled capsids displayed an increase in retrograde motion that prevented efficient delivery of capsids to the distal axon. The aberrant increase in retrograde motion was accompanied by loss of a viral membrane marker from the transported capsids, indicating that the viral kinases allow for efficient anterograde transport by stabilizing membrane-capsid interactions during the long transit from the neuron cell body to the distal axon.
Figures
Similar articles
-
Herpes Simplex Virus gE/gI and US9 Promote both Envelopment and Sorting of Virus Particles in the Cytoplasm of Neurons, Two Processes That Precede Anterograde Transport in Axons.J Virol. 2017 May 12;91(11):e00050-17. doi: 10.1128/JVI.00050-17. Print 2017 Jun 1. J Virol. 2017. PMID: 28331094 Free PMC article.
-
Targeting of herpesvirus capsid transport in axons is coupled to association with specific sets of tegument proteins.Proc Natl Acad Sci U S A. 2005 Apr 19;102(16):5832-7. doi: 10.1073/pnas.0500803102. Epub 2005 Mar 28. Proc Natl Acad Sci U S A. 2005. PMID: 15795370 Free PMC article.
-
Bovine Herpesvirus 1 Invasion of Sensory Neurons by Retrograde Axonal Transport Is Dependent on the pUL37 Region 2 Effector.J Virol. 2022 May 11;96(9):e0148621. doi: 10.1128/jvi.01486-21. Epub 2022 Apr 14. J Virol. 2022. PMID: 35420461 Free PMC article.
-
Anterograde transport of α-herpesviruses in neuronal axons.Virology. 2021 Jul;559:65-73. doi: 10.1016/j.virol.2021.02.011. Epub 2021 Mar 4. Virology. 2021. PMID: 33836340 Review.
-
Transport and egress of herpes simplex virus in neurons.Rev Med Virol. 2008 Jan-Feb;18(1):35-51. doi: 10.1002/rmv.560. Rev Med Virol. 2008. PMID: 17992661 Review.
Cited by
-
The Herpes Simplex Virus 1 Deamidase Enhances Propagation but Is Dispensable for Retrograde Axonal Transport into the Nervous System.J Virol. 2019 Oct 29;93(22):e01172-19. doi: 10.1128/JVI.01172-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31462572 Free PMC article.
-
Herpes simplex virus utilizes the large secretory vesicle pathway for anterograde transport of tegument and envelope proteins and for viral exocytosis from growth cones of human fetal axons.J Virol. 2009 Apr;83(7):3187-99. doi: 10.1128/JVI.01579-08. Epub 2009 Jan 28. J Virol. 2009. PMID: 19176621 Free PMC article.
-
Peroxiredoxin 1 Interacts with TBK1/IKKε and Negatively Regulates Pseudorabies Virus Propagation by Promoting Innate Immunity.J Virol. 2021 Sep 9;95(19):e0092321. doi: 10.1128/JVI.00923-21. Epub 2021 Sep 9. J Virol. 2021. PMID: 34260286 Free PMC article.
-
Resolving the assembly state of herpes simplex virus during axon transport by live-cell imaging.J Virol. 2010 Dec;84(24):13019-30. doi: 10.1128/JVI.01296-10. Epub 2010 Sep 1. J Virol. 2010. PMID: 20810730 Free PMC article.
-
Alphaherpesvirus US3 protein-mediated inhibition of the m6A mRNA methyltransferase complex.Cell Rep. 2022 Jul 19;40(3):111107. doi: 10.1016/j.celrep.2022.111107. Cell Rep. 2022. PMID: 35858564 Free PMC article.
References
-
- Coulter LJ, Moss HW, Lang J, McGeoch DJ. A mutant of herpes simplex virus type 1 in which the UL13 protein kinase gene is disrupted. J Gen Virol. 1993;74(Pt 3):387–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

