Akt phosphorylation and nuclear phosphoinositide association mediate mRNA export and cell proliferation activities by ALY
- PMID: 18562279
- PMCID: PMC2438396
- DOI: 10.1073/pnas.0802533105
Akt phosphorylation and nuclear phosphoinositide association mediate mRNA export and cell proliferation activities by ALY
Abstract
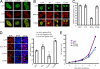
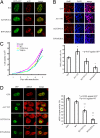
Nuclear PI3K and its downstream effectors play essential roles in a variety of cellular activities including cell proliferation, survival, differentiation, and pre-mRNA splicing. Aly is a nuclear speckle protein implicated in mRNA export. Here we show that Aly is a physiological target of nuclear PI3K signaling, which regulates its subnuclear residency, cell proliferation, and mRNA export activities through nuclear Akt phosphorylation and phosphoinositide association. Nuclear Akt phosphorylates Aly on threonine-219, which is required for its interaction with Akt. Aly binds phosphoinositides, and this action is regulated by Akt-mediated phosphorylation. Phosphoinositide binding but not Akt phosphorylation dictates Aly's nuclear speckle residency. Depletion of Aly results in cell growth suppression and mRNA export reduction. Inhibition of Aly phosphorylation substantially decreases cell proliferation and mRNA export. Furthermore, disruption of phosphoinositide association with Aly also significantly reduces these activities. Thus, nuclear PI3K signaling mediates both cell proliferation and mRNA export functions of Aly.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Nuclear phosphoinositide signaling regulates messenger RNA export.RNA Biol. 2009 Jan-Mar;6(1):12-6. doi: 10.4161/rna.6.1.7439. Epub 2009 Jan 19. RNA Biol. 2009. PMID: 19106628 Free PMC article. Review.
-
Regulation of mRNA export by the PI3 kinase/AKT signal transduction pathway.Mol Biol Cell. 2013 Apr;24(8):1208-21. doi: 10.1091/mbc.E12-06-0450. Epub 2013 Feb 20. Mol Biol Cell. 2013. PMID: 23427269 Free PMC article.
-
The protein Aly links pre-messenger-RNA splicing to nuclear export in metazoans.Nature. 2000 Sep 21;407(6802):401-5. doi: 10.1038/35030160. Nature. 2000. PMID: 11014198
-
Pre-mRNA splicing and mRNA export linked by direct interactions between UAP56 and Aly.Nature. 2001 Oct 11;413(6856):644-7. doi: 10.1038/35098106. Nature. 2001. PMID: 11675789
-
A new view of mRNA export: separating the wheat from the chaff.Nat Cell Biol. 2001 Sep;3(9):E201-4. doi: 10.1038/ncb0901-e201. Nat Cell Biol. 2001. PMID: 11533670 Review.
Cited by
-
Export of adenoviral late mRNA from the nucleus requires the Nxf1/Tap export receptor.J Virol. 2011 Feb;85(4):1429-38. doi: 10.1128/JVI.02108-10. Epub 2010 Dec 1. J Virol. 2011. PMID: 21123381 Free PMC article.
-
Nuclear phosphoinositide signaling regulates messenger RNA export.RNA Biol. 2009 Jan-Mar;6(1):12-6. doi: 10.4161/rna.6.1.7439. Epub 2009 Jan 19. RNA Biol. 2009. PMID: 19106628 Free PMC article. Review.
-
The nuclear protein ALY binds to and modulates the activity of transcription factor E2F2.Mol Cell Proteomics. 2013 May;12(5):1087-98. doi: 10.1074/mcp.M112.024158. Epub 2013 Jan 7. Mol Cell Proteomics. 2013. PMID: 23297349 Free PMC article.
-
Defective phosphoinositide metabolism in autism.J Neurosci Res. 2017 May;95(5):1161-1173. doi: 10.1002/jnr.23797. Epub 2016 Jul 4. J Neurosci Res. 2017. PMID: 27376697 Free PMC article. Review.
-
PIP kinases define PI4,5P₂signaling specificity by association with effectors.Biochim Biophys Acta. 2015 Jun;1851(6):711-23. doi: 10.1016/j.bbalip.2015.01.009. Epub 2015 Jan 21. Biochim Biophys Acta. 2015. PMID: 25617736 Free PMC article. Review.
References
-
- Cocco L, Martelli AM, Gilmour RS, Rhee SG, Manzoli FA. Nuclear phospholipase C and signaling. Biochim Biophys Acta. 2001;1530:1–14. - PubMed
-
- Ye K, et al. Phospholipase C gamma 1 is a physiological guanine nucleotide exchange factor for the nuclear GTPase PIKE. Nature. 2002;415:541–544. - PubMed
-
- Zhang X, et al. Phosphatidylinositol-4-phosphate 5-kinase isozymes catalyze the synthesis of 3-phosphate-containing phosphatidylinositol signaling molecules. J Biol Chem. 1997;272:17756–17761. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

