Transforming growth factor-beta receptor blockade augments the effectiveness of adoptive T-cell therapy of established solid cancers
- PMID: 18559619
- PMCID: PMC2491721
- DOI: 10.1158/1078-0432.CCR-08-0356
Transforming growth factor-beta receptor blockade augments the effectiveness of adoptive T-cell therapy of established solid cancers
Abstract
Purpose: Adoptive cellular immunotherapy is a promising approach to eradicate established tumors. However, a significant hurdle in the success of cellular immunotherapy involves recently identified mechanisms of immune suppression on cytotoxic T cells at the effector phase. Transforming growth factor-beta (TGF-beta) is one of the most important of these immunosuppressive factors because it affects both T-cell and macrophage functions. We thus hypothesized that systemic blockade of TGF-beta signaling combined with adoptive T-cell transfer would enhance the effectiveness of the therapy.
Experimental design: Flank tumors were generated in mice using the chicken ovalbumin-expressing thymoma cell line, EG7. Splenocytes from transgenic OT-1 mice (whose CD8 T cells recognize an immunodominant peptide in chicken ovalbumin) were activated in vitro and adoptively transferred into mice bearing large tumors in the presence or absence of an orally available TGF-beta receptor-I kinase blocker (SM16).
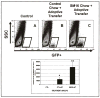
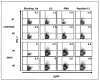
Results: We observed markedly smaller tumors in the group receiving the combination of SM16 chow and adoptive transfer. Additional investigation revealed that TGF-beta receptor blockade increased the persistence of adoptively transferred T cells in the spleen and lymph nodes, increased numbers of adoptively transferred T cells within tumors, increased activation of these infiltrating T cells, and altered the tumor microenvironment with a significant increase in tumor necrosis factor-alpha and decrease in arginase mRNA expression.
Conclusions: We found that systemic blockade of TGF-beta receptor activity augmented the antitumor activity of adoptively transferred T cells and may thus be a useful adjunct in future clinical trials.
Figures


Similar articles
-
Systemic blockade of transforming growth factor-beta signaling augments the efficacy of immunogene therapy.Cancer Res. 2008 Dec 15;68(24):10247-56. doi: 10.1158/0008-5472.CAN-08-1494. Cancer Res. 2008. PMID: 19074893 Free PMC article.
-
Adoptive transfer of tumor-reactive transforming growth factor-beta-insensitive CD8+ T cells: eradication of autologous mouse prostate cancer.Cancer Res. 2005 Mar 1;65(5):1761-9. doi: 10.1158/0008-5472.CAN-04-3169. Cancer Res. 2005. PMID: 15753372
-
Reinforcement of cancer immunotherapy by adoptive transfer of cblb-deficient CD8+ T cells combined with a DC vaccine.Immunol Cell Biol. 2012 Jan;90(1):130-4. doi: 10.1038/icb.2011.11. Epub 2011 Mar 8. Immunol Cell Biol. 2012. PMID: 21383769
-
Harnessing the effect of adoptively transferred tumor-reactive T cells on endogenous (host-derived) antitumor immunity.Clin Dev Immunol. 2010;2010:139304. doi: 10.1155/2010/139304. Epub 2010 Nov 7. Clin Dev Immunol. 2010. PMID: 21076522 Free PMC article. Review.
-
Metabolic Challenges in Anticancer CD8 T Cell Functions.Immune Netw. 2023 Jan 27;23(1):e9. doi: 10.4110/in.2023.23.e9. eCollection 2023 Feb. Immune Netw. 2023. PMID: 36911801 Free PMC article. Review.
Cited by
-
The role of the immune system in non-small cell lung carcinoma and potential for therapeutic intervention.Transl Lung Cancer Res. 2015 Apr;4(2):177-90. doi: 10.3978/j.issn.2218-6751.2015.01.11. Transl Lung Cancer Res. 2015. PMID: 25870800 Free PMC article. Review.
-
The small molecule TGF-β signaling inhibitor SM16 synergizes with agonistic OX40 antibody to suppress established mammary tumors and reduce spontaneous metastasis.Cancer Immunol Immunother. 2012 Apr;61(4):511-21. doi: 10.1007/s00262-011-1119-y. Epub 2011 Oct 5. Cancer Immunol Immunother. 2012. PMID: 21971588 Free PMC article.
-
Chimeric-antigen receptor T (CAR-T) cell therapy for solid tumors: challenges and opportunities.Oncotarget. 2017 Jul 18;8(52):90521-90531. doi: 10.18632/oncotarget.19361. eCollection 2017 Oct 27. Oncotarget. 2017. PMID: 29163850 Free PMC article. Review.
-
TGFβ biology in cancer progression and immunotherapy.Nat Rev Clin Oncol. 2021 Jan;18(1):9-34. doi: 10.1038/s41571-020-0403-1. Epub 2020 Jul 24. Nat Rev Clin Oncol. 2021. PMID: 32710082 Free PMC article. Review.
-
Delivery technologies for cancer immunotherapy.Nat Rev Drug Discov. 2019 Mar;18(3):175-196. doi: 10.1038/s41573-018-0006-z. Nat Rev Drug Discov. 2019. PMID: 30622344 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

