Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages
- PMID: 18552351
- PMCID: PMC2516976
- DOI: 10.1074/jbc.M801408200
Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages
Abstract
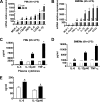
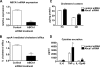
Macrophage-specific Abca1 knock-out (Abca1(-)(M)(/-)(M)) mice were generated to determine the role of macrophage ABCA1 expression in plasma lipoprotein concentrations and the innate immune response of macrophages. Plasma lipid and lipoprotein concentrations in chow-fed Abca1(-)(M)(/-)(M) and wild-type (WT) mice were indistinguishable. Compared with WT macrophages, Abca1(-)(M)(/-)(M) macrophages had a >95% reduction in ABCA1 protein, failed to efflux lipid to apoA-I, and had a significant increase in free cholesterol (FC) and membrane lipid rafts without induction of endoplasmic reticulum stress. Lipopolysaccharide (LPS)-treated Abca1(-)(M)(/-)(M) macrophages exhibited enhanced expression of pro-inflammatory cytokines and increased activation of the NF-kappaB and MAPK pathways, which could be diminished by silencing MyD88 or by chemical inhibition of NF-kappaB or MAPK. In vivo LPS injection also resulted in a higher pro-inflammatory response in Abca1(-)(M)(/-)(M) mice compared with WT mice. Furthermore, cholesterol depletion of macrophages with methyl-beta-cyclodextrin normalized FC content between the two genotypes and their response to LPS; cholesterol repletion of macrophages resulted in increased cellular FC accumulation and enhanced cellular response to LPS. Our results suggest that macrophage ABCA1 expression may protect against atherosclerosis by facilitating the net removal of excess lipid from macrophages and dampening pro-inflammatory MyD88-dependent signaling pathways by reduction of cell membrane FC and lipid raft content.
Figures
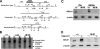




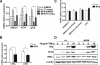
Similar articles
-
Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol.J Lipid Res. 2010 Nov;51(11):3196-206. doi: 10.1194/jlr.M006486. Epub 2010 Jul 21. J Lipid Res. 2010. PMID: 20650929 Free PMC article.
-
Myeloid cell-specific ABCA1 deletion protects mice from bacterial infection.Circ Res. 2012 Nov 9;111(11):1398-409. doi: 10.1161/CIRCRESAHA.112.269043. Epub 2012 Sep 6. Circ Res. 2012. PMID: 22955730 Free PMC article.
-
Apolipoprotein A-I inhibits CD40 proinflammatory signaling via ATP-binding cassette transporter A1-mediated modulation of lipid raft in macrophages.J Atheroscler Thromb. 2012;19(9):823-36. doi: 10.5551/jat.12823. Epub 2012 Jul 4. J Atheroscler Thromb. 2012. PMID: 22786446
-
ATP-binding cassette transporters A1 and G1, HDL metabolism, cholesterol efflux, and inflammation: important targets for the treatment of atherosclerosis.Curr Drug Targets. 2011 May;12(5):647-60. doi: 10.2174/138945011795378522. Curr Drug Targets. 2011. PMID: 21039336 Review.
-
The cell cholesterol exporter ABCA1 as a protector from cardiovascular disease and diabetes.Biochim Biophys Acta. 2009 Jul;1791(7):563-72. doi: 10.1016/j.bbalip.2009.03.011. Epub 2009 Apr 1. Biochim Biophys Acta. 2009. PMID: 19344785 Review.
Cited by
-
Mammalian P4-ATPases and ABC transporters and their role in phospholipid transport.Biochim Biophys Acta. 2013 Mar;1831(3):555-74. doi: 10.1016/j.bbalip.2012.10.006. Epub 2012 Oct 26. Biochim Biophys Acta. 2013. PMID: 23103747 Free PMC article. Review.
-
MicroRNA-mediated mechanisms of the cellular stress response in atherosclerosis.Nat Rev Cardiol. 2015 Jun;12(6):361-74. doi: 10.1038/nrcardio.2015.38. Epub 2015 Apr 7. Nat Rev Cardiol. 2015. PMID: 25855604 Review.
-
Crosstalk Between LXR and Caveolin-1 Signaling Supports Cholesterol Efflux and Anti-Inflammatory Pathways in Macrophages.Front Endocrinol (Lausanne). 2021 May 27;12:635923. doi: 10.3389/fendo.2021.635923. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34122329 Free PMC article.
-
Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses.Arterioscler Thromb Vasc Biol. 2010 Feb;30(2):139-43. doi: 10.1161/ATVBAHA.108.179283. Epub 2009 Oct 1. Arterioscler Thromb Vasc Biol. 2010. PMID: 19797709 Free PMC article. Review.
-
miR-223 Exerts Translational Control of Proatherogenic Genes in Macrophages.Circ Res. 2022 Jun 24;131(1):42-58. doi: 10.1161/CIRCRESAHA.121.319120. Epub 2022 May 25. Circ Res. 2022. PMID: 35611698 Free PMC article.
References
-
- Langmann, T., Klucken, J., Reil, M., Liebisch, G., Luciani, M. F., Chimini, G., Kaminski, W. E., and Schmitz, G. (1999) Biochem. Biophys. Res. Commun. 257 29–33 - PubMed
-
- Wellington, C. L., Walker, E. K., Suarez, A., Kwok, A., Bissada, N., Singaraja, R., Yang, Y. Z., Zhang, L.-H., James, E., Wilson, J. E., Francone, O., McManus, B. M., and Hayden, M. R. (2002) Lab. Investig. 82 273–283 - PubMed
-
- Oram, J. F., and Lawn, R. M. (2001) J. Lipid Res. 42 1173–1179 - PubMed
-
- Attie, A. D., Kastelein, J. P. P., and Hayden, M. R. (2001) J. Lipid Res. 42 1717–1726 - PubMed
-
- Bodzioch, M., Orsó, E., Klucken, J., Langmann, T., Böttcher, A., Diederich, W., Drobnik, W., Barlage, S., Buchler, C., Porsch-Ozcurumez, M., Kaminski, W. E., Hahmann, H. W., Oette, K., Rothe, G., Aslanidis, C., Lackner, K. J., and Schmitz, G. (1999) Nat. Genet. 22 347–351 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

