Elevated glutathione levels confer cellular sensitization to cisplatin toxicity by up-regulation of copper transporter hCtr1
- PMID: 18523133
- PMCID: PMC2583459
- DOI: 10.1124/mol.108.047969
Elevated glutathione levels confer cellular sensitization to cisplatin toxicity by up-regulation of copper transporter hCtr1
Abstract
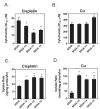
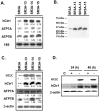
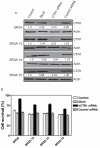
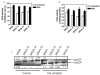
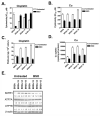
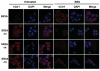
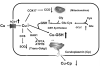
Previous studies have demonstrated that treating cultured cells with cisplatin (CDDP) up-regulated the expression of glutathione (GSH) and its de novo rate-limiting enzyme glutamate-cysteine ligase (GCL), which consists of a catalytic (GCLC) and a modifier (GCLM) subunit. It has also been shown that many CDDP-resistant cell lines exhibit high levels of GCLC/GCLM and GSH. Because the GSH system is the major intracellular regulator of redox conditions that serve as an important detoxification cytoprotector, these results have been taken into consideration that elevated levels of GCL/GSH are responsible for the CDDP resistance. In contrast to this context, we demonstrated here that overexpression of GSH by transfection with an expression plasmid containing the GCLC cDNA conferred sensitization to CDDP through up-regulation of human copper transporter (hCtr) 1, which is also a transporter for CDDP. Depleting GSH levels in these transfected cells reversed CDDP sensitivity with concomitant reduction of hCtr1 expression. Although rates of copper transport were also up-regulated in the transfected cells, these cells exhibited biochemical signature of copper deficiency, suggesting that GSH functions as an intracellular copper-chelator and that overexpression of GSH can alter copper metabolism. More importantly, our results reveal a new role of GSH in the regulation of CDDP sensitivity. Overproduction of GSH depletes the bioavailable copper pool, leading to up-regulation of hCtr1 and sensitization of CDDP transport and cell killing. These findings also have important implications in that modulation of the intracellular copper pool may be a novel strategy for improving chemotherapeutic efficacy of platinum-based antitumor agents.
Figures
Similar articles
-
Mechanistic basis for overcoming platinum resistance using copper chelating agents.Mol Cancer Ther. 2012 Nov;11(11):2483-94. doi: 10.1158/1535-7163.MCT-12-0580. Epub 2012 Aug 21. Mol Cancer Ther. 2012. PMID: 22914438 Free PMC article.
-
Elevated GSH level increases cadmium resistance through down-regulation of Sp1-dependent expression of the cadmium transporter ZIP8.Mol Pharmacol. 2008 Sep;74(3):823-33. doi: 10.1124/mol.108.046862. Epub 2008 Jun 12. Mol Pharmacol. 2008. PMID: 18556457 Free PMC article.
-
Role of glutathione in the regulation of Cisplatin resistance in cancer chemotherapy.Met Based Drugs. 2010;2010:430939. doi: 10.1155/2010/430939. Epub 2010 Sep 14. Met Based Drugs. 2010. PMID: 20885916 Free PMC article.
-
Overcoming platinum drug resistance with copper-lowering agents.Anticancer Res. 2013 Oct;33(10):4157-61. Anticancer Res. 2013. PMID: 24122978 Free PMC article. Review.
-
Targeting drug transport mechanisms for improving platinum-based cancer chemotherapy.Expert Opin Ther Targets. 2015;19(10):1307-17. doi: 10.1517/14728222.2015.1043269. Epub 2015 May 25. Expert Opin Ther Targets. 2015. PMID: 26004625 Free PMC article. Review.
Cited by
-
The relationship of thioredoxin-1 and cisplatin resistance: its impact on ROS and oxidative metabolism in lung cancer cells.Mol Cancer Ther. 2012 Mar;11(3):604-15. doi: 10.1158/1535-7163.MCT-11-0599. Epub 2012 Jan 16. Mol Cancer Ther. 2012. PMID: 22248473 Free PMC article.
-
Predictive and prognostic value of human copper transporter 1 (hCtr1) in patients with stage III non-small-cell lung cancer receiving first-line platinum-based doublet chemotherapy.Lung Cancer. 2012 Feb;75(2):228-34. doi: 10.1016/j.lungcan.2011.06.011. Epub 2011 Jul 23. Lung Cancer. 2012. PMID: 21788094 Free PMC article.
-
Esterase-Responsive Polyglycerol-Based Nanogels for Intracellular Drug Delivery in Rare Gastrointestinal Stromal Tumors.Pharmaceuticals (Basel). 2023 Nov 16;16(11):1618. doi: 10.3390/ph16111618. Pharmaceuticals (Basel). 2023. PMID: 38004483 Free PMC article.
-
Mechanistic basis for overcoming platinum resistance using copper chelating agents.Mol Cancer Ther. 2012 Nov;11(11):2483-94. doi: 10.1158/1535-7163.MCT-12-0580. Epub 2012 Aug 21. Mol Cancer Ther. 2012. PMID: 22914438 Free PMC article.
-
The glutamate transport inhibitor DL-Threo-β-Benzyloxyaspartic acid (DL-TBOA) differentially affects SN38- and oxaliplatin-induced death of drug-resistant colorectal cancer cells.BMC Cancer. 2015 May 16;15:411. doi: 10.1186/s12885-015-1405-8. BMC Cancer. 2015. PMID: 25981639 Free PMC article.
References
-
- Chen J, Liao C, Mao SJ, Chen T, Weng C. A Simple Technique for the Simultaneous Determination of Molecular Weight and Activity of Superoxide Dismutase Using SDS-PAGE. J Biochem Biophys Methods. 2001;47:233–237. - PubMed
-
- Cobine PA, Pierrel F, Winge DR. Copper Trafficking to the Mitochondrion and Assembly of Copper Metalloenzymes. Biochim Biophys Acta. 2006;1763:759–772. - PubMed
-
- Cole SP, Sparks KE, Fraser K, Loe DW, Grant CE, Wilson GM, Deeley RG. Pharmacological Characterization of Multidrug Resistant MRP-Transfected Human Tumor Cells. Cancer Res. 1994;54:5902–5910. - PubMed
-
- Das SK, Ray K. Wilson’s Disease: an Update. Nat Clin Pract Neurol. 2006;2:482–493. - PubMed
-
- Eisses JF, Chi Y, Kaplan JH. Stable Plasma Membrane Levels of HCTR1 Mediate Cellular Copper Uptake. J Biol Chem. 2005;280:9635–9639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
