The secretion inhibitor Exo2 perturbs trafficking of Shiga toxin between endosomes and the trans-Golgi network
- PMID: 18522538
- PMCID: PMC2552392
- DOI: 10.1042/BJ20080149
The secretion inhibitor Exo2 perturbs trafficking of Shiga toxin between endosomes and the trans-Golgi network
Abstract
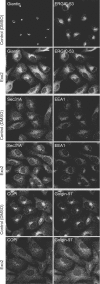
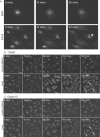
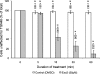
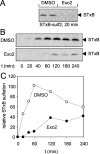
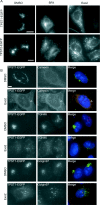
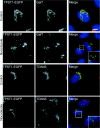
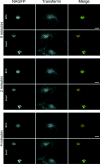
The small-molecule inhibitor Exo2 {4-hydroxy-3-methoxy-(5,6,7,8-tetrahydrol[1]benzothieno[2,3-d]pyrimidin-4-yl)hydraz-one benzaldehyde} has been reported to disrupt the Golgi apparatus completely and to stimulate Golgi-ER (endoplasmic reticulum) fusion in mammalian cells, akin to the well-characterized fungal toxin BFA (brefeldin A). It has also been reported that Exo2 does not affect the integrity of the TGN (trans-Golgi network), or the direct retrograde trafficking of the glycolipid-binding cholera toxin from the TGN to the ER lumen. We have examined the effects of BFA and Exo2, and found that both compounds are indistinguishable in their inhibition of anterograde transport and that both reagents significantly disrupt the morphology of the TGN in HeLa and in BS-C-1 cells. However, Exo2, unlike BFA, does not induce tubulation and merging of the TGN and endosomal compartments. Furthermore, and in contrast with its effects on cholera toxin, Exo2 significantly perturbs the delivery of Shiga toxin to the ER. Together, these results suggest that the likely target(s) of Exo2 operate at the level of the TGN, the Golgi and a subset of early endosomes, and thus Exo2 provides a more selective tool than BFA for examining membrane trafficking in mammalian cells.
Figures


Similar articles
-
An Exo2 derivative affects ER and Golgi morphology and vacuolar sorting in a tissue-specific manner in arabidopsis.Traffic. 2011 Nov;12(11):1552-62. doi: 10.1111/j.1600-0854.2011.01258.x. Epub 2011 Aug 24. Traffic. 2011. PMID: 21801289
-
Retrograde transport of cholera toxin from the plasma membrane to the endoplasmic reticulum requires the trans-Golgi network but not the Golgi apparatus in Exo2-treated cells.EMBO Rep. 2004 Jun;5(6):596-601. doi: 10.1038/sj.embor.7400152. Epub 2004 May 21. EMBO Rep. 2004. PMID: 15153932 Free PMC article.
-
Fine tuning Exo2, a small molecule inhibitor of secretion and retrograde trafficking pathways in mammalian cells.Mol Biosyst. 2010 Oct;6(10):2030-8. doi: 10.1039/c0mb00035c. Epub 2010 Aug 9. Mol Biosyst. 2010. PMID: 20697620
-
Transport of protein toxins into cells: pathways used by ricin, cholera toxin and Shiga toxin.FEBS Lett. 2002 Oct 2;529(1):49-53. doi: 10.1016/s0014-5793(02)03182-4. FEBS Lett. 2002. PMID: 12354612 Review.
-
Alternate routes for drug delivery to the cell interior: pathways to the Golgi apparatus and endoplasmic reticulum.Adv Drug Deliv Rev. 2007 Aug 10;59(8):782-97. doi: 10.1016/j.addr.2007.06.006. Epub 2007 Jun 28. Adv Drug Deliv Rev. 2007. PMID: 17669543 Free PMC article. Review.
Cited by
-
Syntaxin 5-dependent retrograde transport to the trans-Golgi network is required for adeno-associated virus transduction.J Virol. 2015 Feb;89(3):1673-87. doi: 10.1128/JVI.02520-14. Epub 2014 Nov 19. J Virol. 2015. PMID: 25410859 Free PMC article.
-
Inhibitors of the cellular trafficking of ricin.Toxins (Basel). 2012 Jan;4(1):15-27. doi: 10.3390/toxins4010015. Epub 2012 Jan 6. Toxins (Basel). 2012. PMID: 22347620 Free PMC article. Review.
-
Identification of a peptide-based neutralizer that potently inhibits both Shiga toxins 1 and 2 by targeting specific receptor-binding regions.Infect Immun. 2013 Jun;81(6):2133-8. doi: 10.1128/IAI.01256-12. Epub 2013 Apr 1. Infect Immun. 2013. PMID: 23545297 Free PMC article.
-
Annexin A1 and A2: roles in retrograde trafficking of Shiga toxin.PLoS One. 2012;7(7):e40429. doi: 10.1371/journal.pone.0040429. Epub 2012 Jul 6. PLoS One. 2012. PMID: 22792315 Free PMC article.
-
Drugging the Small GTPase Pathways in Cancer Treatment: Promises and Challenges.Cells. 2019 Mar 16;8(3):255. doi: 10.3390/cells8030255. Cells. 2019. PMID: 30884855 Free PMC article. Review.
References
-
- Bonifacino J. S., Glick B. S. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. - PubMed
-
- Schekman R., Novick P. 23 genes, 23 years later. Cell. 2004;116:S13–S15. - PubMed
-
- Orci L., Tagaya M., Amherdt M., Perrelet A., Donaldson J. G., Lippincott-Schwartz J., Klausner R. D., Rothman J. E. Brefeldin A, a drug that blocks secretion, prevents the assembly of non-clathrin-coated buds on Golgi cisternae. Cell. 1991;64:1183–1195. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

