Deregulation of PKN1 activity disrupts neurofilament organisation and axonal transport
- PMID: 18519042
- PMCID: PMC4516414
- DOI: 10.1016/j.febslet.2008.05.034
Deregulation of PKN1 activity disrupts neurofilament organisation and axonal transport
Abstract
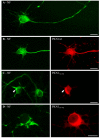
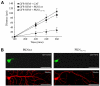
Neurofilaments are synthesised in neuronal cell bodies and then transported through axons. Damage to neurofilament transport is seen in amyotrophic lateral sclerosis (ALS). Here, we show that PKN1, a neurofilament head-rod domain kinase is cleaved and activated in SOD1G93A transgenic mice that are a model of ALS. Moreover, we demonstrate that glutamate, a proposed toxic mechanism in ALS leads to caspase cleavage and disruption of PKN1 in neurons. Finally, we demonstrate that a cleaved form of PKN1 but not wild-type PKN1 disrupts neurofilament organisation and axonal transport. Thus, deregulation of PKN1 may contribute to the pathogenic process in ALS.
Figures



Similar articles
-
Defective neurofilament transport in mouse models of amyotrophic lateral sclerosis: a review.Neurochem Res. 2003 Jul;28(7):1041-7. doi: 10.1023/a:1023259207015. Neurochem Res. 2003. PMID: 12737529 Review.
-
Impairment of axonal transport in the axon hillock and the initial segment of anterior horn neurons in transgenic mice with a G93A mutant SOD1 gene.Acta Neuropathol. 2005 Jul;110(1):48-56. doi: 10.1007/s00401-005-1021-9. Epub 2005 May 26. Acta Neuropathol. 2005. PMID: 15920660
-
Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1.Neurobiol Dis. 2000 Dec;7(6 Pt B):623-43. doi: 10.1006/nbdi.2000.0299. Neurobiol Dis. 2000. PMID: 11114261
-
p38alpha stress-activated protein kinase phosphorylates neurofilaments and is associated with neurofilament pathology in amyotrophic lateral sclerosis.Mol Cell Neurosci. 2004 Jun;26(2):354-64. doi: 10.1016/j.mcn.2004.02.009. Mol Cell Neurosci. 2004. PMID: 15207859
-
Superoxide dismutase and neurofilament transgenic models of amyotrophic lateral sclerosis.J Exp Zool. 1998 Sep-Oct 1;282(1-2):32-47. J Exp Zool. 1998. PMID: 9723164 Review.
Cited by
-
PKN1 Exerts Neurodegenerative Effects in an In Vitro Model of Cerebellar Hypoxic-Ischemic Encephalopathy via Inhibition of AKT/GSK3β Signaling.Biomolecules. 2023 Oct 31;13(11):1599. doi: 10.3390/biom13111599. Biomolecules. 2023. PMID: 38002281 Free PMC article.
-
APOE4 confers transcriptomic and functional alterations to primary mouse microglia.Neurobiol Dis. 2022 Mar;164:105615. doi: 10.1016/j.nbd.2022.105615. Epub 2022 Jan 11. Neurobiol Dis. 2022. PMID: 35031484 Free PMC article.
-
Neuromodulatory Effects of Guanine-Based Purines in Health and Disease.Front Cell Neurosci. 2018 Oct 23;12:376. doi: 10.3389/fncel.2018.00376. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30459558 Free PMC article. Review.
-
Protein kinase N1 critically regulates cerebellar development and long-term function.J Clin Invest. 2018 May 1;128(5):2076-2088. doi: 10.1172/JCI96165. Epub 2018 Apr 16. J Clin Invest. 2018. PMID: 29494346 Free PMC article.
-
Activation of PKN mediates survival of cardiac myocytes in the heart during ischemia/reperfusion.Circ Res. 2010 Sep 3;107(5):642-9. doi: 10.1161/CIRCRESAHA.110.217554. Epub 2010 Jul 1. Circ Res. 2010. PMID: 20595653 Free PMC article.
References
-
- Mukai H. The structure and function of PKN, a protein kinase having a catalytic domain homologous to that of PKC. J. Biochem. (Tokyo) 2003;133:17–27. - PubMed
-
- Mukai H, Toshimori M, Shibata H, Kitagawa M, Shimakawa M, Miyahara M, Sunakawa H, Ono Y. PKN associates and phosphorylates the head–rod domain of neurofilament protein. J. Biol. Chem. 1996;271:9816–9822. - PubMed
-
- Xiao S, McLean J, Robertson J. Neuronal intermediate filaments and ALS: a new look at an old question. Biochim. Biophys. Acta. 2006;1762:1001–1012. - PubMed
-
- Williamson TL, Cleveland DW. Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nat. Neurosci. 1999;2:50–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

