UNBS5162, a novel naphthalimide that decreases CXCL chemokine expression in experimental prostate cancers
- PMID: 18516294
- PMCID: PMC2386542
- DOI: 10.1593/neo.08290
UNBS5162, a novel naphthalimide that decreases CXCL chemokine expression in experimental prostate cancers
Abstract
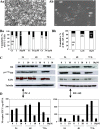
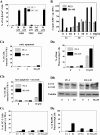
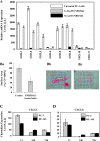
Several naphthalimides have been evaluated clinically as potential anticancer agents. UNBS3157, a naphthalimide that belongs to the same class as amonafide, was designed to avoid the specific activating metabolism that induces amonafide's hematotoxicity. The current study shows that UNBS3157 rapidly and irreversibly hydrolyzes to UNBS5162 without generating amonafide. In vivo UNBS5162 after repeat administration significantly increased survival in orthotopic human prostate cancer models. Results obtained by the National Cancer Institute (NCI) using UNBS3157 and UNBS5162 against the NCI 60 cell line panel did not show a correlation with any other compound present in the NCI database, including amonafide, thereby suggesting a unique mechanism of action for these two novel naphthalimides. Affymetrix genome-wide microarray analysis and enzyme-linked immunosorbent assay revealed that in vitro exposure of PC-3 cells to UNBS5162 (1 microM for 5 successive days) dramatically decreased the expression of the proangiogenic CXCL chemokines. Histopathology additionally revealed antiangiogenic properties in vivo for UNBS5162 in the orthotopic PC-3 model. In conclusion, the present study reveals UNBS5162 to be a pan-antagonist of CXCL chemokine expression, with the compound displaying antitumor effects in experimental models of human refractory prostate cancer when administered alone and found to enhance the activity of taxol when coadministered with the taxoid.
Figures


Similar articles
-
UNBS5162 inhibits the proliferation of esophageal cancer squamous cells via the PI3K/AKT signaling pathway.Mol Med Rep. 2018 Jan;17(1):549-555. doi: 10.3892/mmr.2017.7893. Epub 2017 Oct 26. Mol Med Rep. 2018. PMID: 29115622
-
UNBS5162 inhibits colon cancer growth via suppression of PI3K/Akt signaling pathway.Med Sci (Paris). 2018 Oct;34 Focus issue F1:99-104. doi: 10.1051/medsci/201834f117. Epub 2018 Nov 7. Med Sci (Paris). 2018. PMID: 30403183
-
2,2,2-Trichloro-N-({2-[2-(dimethylamino)ethyl]-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin- 5-yl}carbamoyl)acetamide (UNBS3157), a novel nonhematotoxic naphthalimide derivative with potent antitumor activity.J Med Chem. 2007 Aug 23;50(17):4122-34. doi: 10.1021/jm070315q. Epub 2007 Jul 21. J Med Chem. 2007. PMID: 17658777
-
Recent developments on 1,8-Naphthalimide moiety as potential target for anticancer agents.Bioorg Chem. 2022 Apr;121:105677. doi: 10.1016/j.bioorg.2022.105677. Epub 2022 Feb 12. Bioorg Chem. 2022. PMID: 35202852 Review.
-
l,8-Naphthalimide based DNA intercalators and anticancer agents. A systematic review from 2007 to 2017.Eur J Med Chem. 2018 Nov 5;159:393-422. doi: 10.1016/j.ejmech.2018.09.055. Epub 2018 Sep 24. Eur J Med Chem. 2018. PMID: 30312931 Review.
Cited by
-
Down-regulation of CXCL11 inhibits colorectal cancer cell growth and epithelial-mesenchymal transition.Onco Targets Ther. 2018 Oct 23;11:7333-7343. doi: 10.2147/OTT.S167872. eCollection 2018. Onco Targets Ther. 2018. PMID: 30425523 Free PMC article.
-
UNBS5162 inhibits SKOV3 ovarian cancer cell proliferation by regulating the PI3K/AKT signalling pathway.Oncol Lett. 2019 Mar;17(3):2976-2982. doi: 10.3892/ol.2019.9890. Epub 2019 Jan 4. Oncol Lett. 2019. PMID: 30854075 Free PMC article.
-
Neoplasia: the second decade.Neoplasia. 2008 Dec;10(12):1314-24. doi: 10.1593/neo.81372. Neoplasia. 2008. PMID: 19048110 Free PMC article.
-
UNBS5162 inhibits proliferation of human retinoblastoma cells by promoting cell apoptosis.Onco Targets Ther. 2017 Nov 6;10:5303-5309. doi: 10.2147/OTT.S145518. eCollection 2017. Onco Targets Ther. 2017. PMID: 29158682 Free PMC article.
-
Methoxyethylamino-numonafide is an efficacious and minimally toxic amonafide derivative in murine models of human cancer.Neoplasia. 2011 May;13(5):453-60. doi: 10.1593/neo.101738. Neoplasia. 2011. PMID: 21532886 Free PMC article.
References
-
- Brana MF, Ramos A. Naphthalimides as anti-cancer agents: synthesis and biological activity. Curr Med Chem Anticancer Agents. 2001;1:237–255. - PubMed
-
- Ratain MJ, Mick R, Berezin F, Janisch L, Schilsky RL, Vogelzang NJ, Lane LB. Phase I study of amonafide dosing based on acetylator phenotype. Cancer Res. 1993;53:2304–2308. - PubMed
-
- Ratain MJ, Rosner G, Allen SL, Costanza M, Van Echo DA, Henderson IC, Schilsky RL. Population pharmacodynamic study of amonafide: a cancer and leukemia group B study. J Clin Oncol. 1995;13:741–747. - PubMed
-
- Brana MF, Cacho M, Garcia MA, de Pascual-Teresa B, Ramos A, Acero N, Llinares F, Munoz-Mingarro D, Abradelo C, Rey-Stolle MF, et al. Synthesis, antitumor activity, molecular modeling, and DNA binding properties of a new series of imidazonaphthalimides. J Med Chem. 2002;45:5813–5816. - PubMed
-
- Bailly C, Carrasco C, Joubert A, Bal C, Wattez N, Hildebrand MP, Lansiaux A, Colson P, Houssier C, Cacho M, et al. Chromophore-modified bisnaphthalimides: DNA recognition, topoisomerase inhibition, and cytotoxic properties of two mono- and bisfuronaphthalimides. Biochemistry. 2003;42:4136–4150. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
