Synthesis and potency of novel uracil nucleotides and derivatives as P2Y2 and P2Y6 receptor agonists
- PMID: 18514530
- PMCID: PMC2483329
- DOI: 10.1016/j.bmc.2008.05.013
Synthesis and potency of novel uracil nucleotides and derivatives as P2Y2 and P2Y6 receptor agonists
Abstract
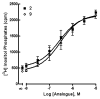
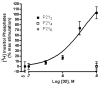
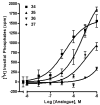
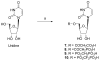
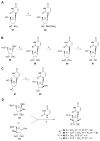
The phosphate, uracil, and ribose moieties of uracil nucleotides were varied structurally for evaluation of agonist activity at the human P2Y(2), P2Y(4), and P2Y(6) receptors. The 2-thio modification, found previously to enhance P2Y(2) receptor potency, could be combined with other favorable modifications to produce novel molecules that exhibit high potencies and receptor selectivities. Phosphonomethylene bridges introduced for stability in analogues of UDP, UTP, and uracil dinucleotides markedly reduced potency. Truncation of dinucleotide agonists of the P2Y(2) receptor, in the form of Up(4)-sugars, indicated that a terminal uracil ring is not essential for moderate potency at this receptor and that specific SAR patterns are observed at this distal end of the molecule. Key compounds reported in this study include 9, alpha,beta-methylene-UDP, a P2Y(6) receptor agonist; 30, Up(4)-phenyl ester and 34, Up(4)-[1]glucose, selective P2Y(2) receptor agonists; dihalomethylene phosphonate analogues 16 and 41, selective P2Y(2) receptor agonists; 43, the 2-thio analogue of INS37217 (P(1)-(uridine-5')-P(4)-(2'-deoxycytidine-5')tetraphosphate), a potent and selective P2Y(2) receptor agonist.
Figures
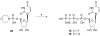
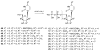
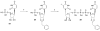

Similar articles
-
Synthesis and structure-activity relationships of uracil nucleotide derivatives and analogues as agonists at human P2Y2, P2Y4, and P2Y6 receptors.J Med Chem. 2006 Nov 30;49(24):7076-87. doi: 10.1021/jm060848j. J Med Chem. 2006. PMID: 17125260
-
Pyrimidine nucleotides with 4-alkyloxyimino and terminal tetraphosphate δ-ester modifications as selective agonists of the P2Y(4) receptor.J Med Chem. 2011 Jun 23;54(12):4018-33. doi: 10.1021/jm101591j. Epub 2011 May 20. J Med Chem. 2011. PMID: 21528910 Free PMC article.
-
Methanocarba modification of uracil and adenine nucleotides: high potency of Northern ring conformation at P2Y1, P2Y2, P2Y4, and P2Y11 but not P2Y6 receptors.J Med Chem. 2002 Jan 3;45(1):208-18. doi: 10.1021/jm010369e. J Med Chem. 2002. PMID: 11754592 Free PMC article.
-
P2 receptors activated by uracil nucleotides--an update.Curr Med Chem. 2006;13(3):289-312. doi: 10.2174/092986706775476052. Curr Med Chem. 2006. PMID: 16475938 Review.
-
Tools and drugs for uracil nucleotide-activated P2Y receptors.Pharmacol Ther. 2018 Oct;190:24-80. doi: 10.1016/j.pharmthera.2018.04.002. Epub 2018 Apr 13. Pharmacol Ther. 2018. PMID: 29660366 Review.
Cited by
-
Nucleoside Tetra- and Pentaphosphates Prepared Using a Tetraphosphorylation Reagent Are Potent Inhibitors of Ribonuclease A.J Am Chem Soc. 2019 Nov 20;141(46):18400-18404. doi: 10.1021/jacs.9b09760. Epub 2019 Nov 11. J Am Chem Soc. 2019. PMID: 31651164 Free PMC article.
-
Investigation of the functional expression of purine and pyrimidine receptors in porcine isolated pancreatic arteries.Purinergic Signal. 2014;10(2):241-9. doi: 10.1007/s11302-013-9403-2. Epub 2013 Dec 6. Purinergic Signal. 2014. PMID: 24310605 Free PMC article.
-
Synthetic Strategies for Dinucleotides Synthesis.Molecules. 2019 Nov 27;24(23):4334. doi: 10.3390/molecules24234334. Molecules. 2019. PMID: 31783537 Free PMC article. Review.
-
Role of the Purinergic P2Y2 Receptor in Pulmonary Hypertension.Int J Environ Res Public Health. 2021 Oct 20;18(21):11009. doi: 10.3390/ijerph182111009. Int J Environ Res Public Health. 2021. PMID: 34769531 Free PMC article. Review.
-
Pyrimidine Nucleotides Containing a (S)-Methanocarba Ring as P2Y6 Receptor Agonists.Medchemcomm. 2017 Oct 1;8(10):1897-1908. doi: 10.1039/C7MD00397H. Epub 2017 Sep 6. Medchemcomm. 2017. PMID: 29423136 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Miscellaneous

