Crucial structural role for the PH and C1 domains of the Vav1 exchange factor
- PMID: 18511940
- PMCID: PMC2427238
- DOI: 10.1038/embor.2008.80
Crucial structural role for the PH and C1 domains of the Vav1 exchange factor
Abstract
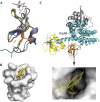
The Vav family of proteins are guanine nucleotide exchange factors (GEFs) for the Rho family of GTPases, which regulate various cellular functions, including T-cell activation. They contain a catalytic Dbl homology (DH) domain that is invariably followed by a pleckstrin homology (PH) domain, which is often required for catalytic activity. Vav proteins are the first GEFs for which an additional C1 domain is required for full biological activity. Here, we present the structure of a Vav1 fragment comprising the DH-PH-C1 domains bound to Rac1. This structure shows that the PH and C1 domains form a single structural unit that packs against the carboxy-terminal helix of the DH domain to stabilize its conformation and to promote nucleotide exchange. In contrast to previous reports, this structure shows that there are no direct contacts between the GTPase and C1 domain but instead suggests new mechanisms for the regulation of Vav1 activity.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Structural basis of guanine nucleotide exchange mediated by the T-cell essential Vav1.J Mol Biol. 2008 Jul 25;380(5):828-43. doi: 10.1016/j.jmb.2008.05.024. Epub 2008 May 17. J Mol Biol. 2008. PMID: 18589439 Free PMC article.
-
Recognition and activation of Rho GTPases by Vav1 and Vav2 guanine nucleotide exchange factors.Biochemistry. 2005 May 3;44(17):6573-85. doi: 10.1021/bi047443q. Biochemistry. 2005. PMID: 15850391
-
The DH and PH domains of Trio coordinately engage Rho GTPases for their efficient activation.J Mol Biol. 2007 May 18;368(5):1307-20. doi: 10.1016/j.jmb.2007.02.060. Epub 2007 Feb 22. J Mol Biol. 2007. PMID: 17391702 Free PMC article.
-
The guanine nucleotide exchange factor Tiam1: a Janus-faced molecule in cellular signaling.Cell Signal. 2014 Mar;26(3):483-91. doi: 10.1016/j.cellsig.2013.11.034. Epub 2013 Dec 2. Cell Signal. 2014. PMID: 24308970 Review.
-
Vav family exchange factors: an integrated regulatory and functional view.Small GTPases. 2014;5(2):9. doi: 10.4161/21541248.2014.973757. Small GTPases. 2014. PMID: 25483299 Free PMC article. Review.
Cited by
-
Signaling by the phosphoinositide 3-kinase family in immune cells.Annu Rev Immunol. 2013;31:675-704. doi: 10.1146/annurev-immunol-032712-095946. Epub 2013 Jan 16. Annu Rev Immunol. 2013. PMID: 23330955 Free PMC article. Review.
-
Mutation of Vav1 adaptor region reveals a new oncogenic activation.Oncotarget. 2015 Feb 10;6(4):2524-37. doi: 10.18632/oncotarget.2629. Oncotarget. 2015. PMID: 25426554 Free PMC article.
-
Liposome reconstitution and modulation of recombinant prenylated human Rac1 by GEFs, GDI1 and Pak1.PLoS One. 2014 Jul 11;9(7):e102425. doi: 10.1371/journal.pone.0102425. eCollection 2014. PLoS One. 2014. PMID: 25014207 Free PMC article.
-
The C1 domain of Vav3, a novel potential therapeutic target.Cell Signal. 2017 Dec;40:133-142. doi: 10.1016/j.cellsig.2017.09.008. Epub 2017 Sep 18. Cell Signal. 2017. PMID: 28927664 Free PMC article.
-
Structural basis for autoinhibition of the guanine nucleotide exchange factor FARP2.Structure. 2013 Mar 5;21(3):355-64. doi: 10.1016/j.str.2013.01.001. Epub 2013 Jan 31. Structure. 2013. PMID: 23375260 Free PMC article.
References
-
- Abe K, Rossman KL, Liu B, Ritola KD, Chiang D, Campbell SL, Burridge K, Der CJ (2000) Vav2 is an activator of Cdc42, Rac1, and RhoA. J Biol Chem 275: 10141–10149 - PubMed
-
- Aghazadeh B, Lowry WE, Huang XY, Rosen MK (2000) Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell 102: 625–633 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

