A cytosolic iron chaperone that delivers iron to ferritin
- PMID: 18511687
- PMCID: PMC2505357
- DOI: 10.1126/science.1157643
A cytosolic iron chaperone that delivers iron to ferritin
Abstract
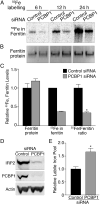
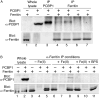
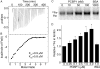
Ferritins are the main iron storage proteins found in animals, plants, and bacteria. The capacity to store iron in ferritin is essential for life in mammals, but the mechanism by which cytosolic iron is delivered to ferritin is unknown. Human ferritins expressed in yeast contain little iron. Human poly (rC)-binding protein 1 (PCBP1) increased the amount of iron loaded into ferritin when expressed in yeast. PCBP1 bound to ferritin in vivo and bound iron and facilitated iron loading into ferritin in vitro. Depletion of PCBP1 in human cells inhibited ferritin iron loading and increased cytosolic iron pools. Thus, PCBP1 can function as a cytosolic iron chaperone in the delivery of iron to ferritin.
Figures

Similar articles
-
Activation of the HIF prolyl hydroxylase by the iron chaperones PCBP1 and PCBP2.Cell Metab. 2011 Nov 2;14(5):647-57. doi: 10.1016/j.cmet.2011.08.015. Cell Metab. 2011. PMID: 22055506 Free PMC article.
-
Iron chaperones PCBP1 and PCBP2 mediate the metallation of the dinuclear iron enzyme deoxyhypusine hydroxylase.Proc Natl Acad Sci U S A. 2014 Jun 3;111(22):8031-6. doi: 10.1073/pnas.1402732111. Epub 2014 May 19. Proc Natl Acad Sci U S A. 2014. PMID: 24843120 Free PMC article.
-
Each member of the poly-r(C)-binding protein 1 (PCBP) family exhibits iron chaperone activity toward ferritin.J Biol Chem. 2013 Jun 14;288(24):17791-802. doi: 10.1074/jbc.M113.460253. Epub 2013 May 2. J Biol Chem. 2013. PMID: 23640898 Free PMC article.
-
Cytosolic iron chaperones: Proteins delivering iron cofactors in the cytosol of mammalian cells.J Biol Chem. 2017 Aug 4;292(31):12764-12771. doi: 10.1074/jbc.R117.791962. Epub 2017 Jun 14. J Biol Chem. 2017. PMID: 28615454 Free PMC article. Review.
-
The flux of iron through ferritin in erythrocyte development.Curr Opin Hematol. 2018 May;25(3):183-188. doi: 10.1097/MOH.0000000000000417. Curr Opin Hematol. 2018. PMID: 29461259 Free PMC article. Review.
Cited by
-
Crucial function of vertebrate glutaredoxin 3 (PICOT) in iron homeostasis and hemoglobin maturation.Mol Biol Cell. 2013 Jun;24(12):1895-903. doi: 10.1091/mbc.E12-09-0648. Epub 2013 Apr 24. Mol Biol Cell. 2013. PMID: 23615448 Free PMC article.
-
Iron imbalance in neurodegeneration.Mol Psychiatry. 2024 Apr;29(4):1139-1152. doi: 10.1038/s41380-023-02399-z. Epub 2024 Jan 12. Mol Psychiatry. 2024. PMID: 38212377 Free PMC article. Review.
-
Iron homeostasis regulates the activity of the microRNA pathway through poly(C)-binding protein 2.Cell Metab. 2012 Jun 6;15(6):895-904. doi: 10.1016/j.cmet.2012.04.021. Epub 2012 May 24. Cell Metab. 2012. PMID: 22633452 Free PMC article.
-
Iron Export through the Transporter Ferroportin 1 Is Modulated by the Iron Chaperone PCBP2.J Biol Chem. 2016 Aug 12;291(33):17303-18. doi: 10.1074/jbc.M116.721936. Epub 2016 Jun 14. J Biol Chem. 2016. PMID: 27302059 Free PMC article.
-
Reductive Mobilization of Iron from Intact Ferritin: Mechanisms and Physiological Implication.Pharmaceuticals (Basel). 2018 Nov 5;11(4):120. doi: 10.3390/ph11040120. Pharmaceuticals (Basel). 2018. PMID: 30400623 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

