A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide
- PMID: 18511561
- PMCID: PMC2409384
- DOI: 10.1073/pnas.0802726105
A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide
Abstract
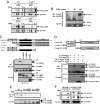
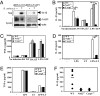
NOD2, a NOD-like receptor (NLR), is an intracellular sensor of bacterial muramyl dipeptide (MDP) that was suggested to promote secretion of the proinflammatory cytokine IL-1beta. Yet, the molecular mechanism by which NOD2 can stimulate IL-1beta secretion, and its biological significance were heretofore unknown. We found that NOD2 through its N-terminal caspase recruitment domain directly binds and activates caspase-1 to trigger IL-1beta processing and secretion in MDP-stimulated macrophages, whereas the C-terminal leucine-rich repeats of NOD2 prevent caspase-1 activation in nonstimulated cells. MDP challenge induces the association of NOD2 with another NLR protein, NALP1, and gel filtration analysis revealed the formation of a complex consisting of NOD2, NALP1, and caspase-1. Importantly, Bacillus anthracis infection induces IL-1beta secretion in a manner that depended on caspase-1 and NOD2. In vitro, Anthrax lethal toxin strongly potentiated IL-1beta secretion, and that response was NOD2 and caspase-1-dependent. Thus, NOD2 plays a key role in the B. anthracis-induced inflammatory response by being a critical mediator of IL-1beta secretion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


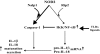
Similar articles
-
Distinct roles for Nod2 protein and autocrine interleukin-1beta in muramyl dipeptide-induced mitogen-activated protein kinase activation and cytokine secretion in human macrophages.J Biol Chem. 2011 Jul 29;286(30):26440-9. doi: 10.1074/jbc.M111.237495. Epub 2011 Jun 9. J Biol Chem. 2011. PMID: 21659536 Free PMC article.
-
Activation of NOD2 in vivo induces IL-1beta production in the eye via caspase-1 but results in ocular inflammation independently of IL-1 signaling.J Leukoc Biol. 2008 Aug;84(2):529-36. doi: 10.1189/jlb.0108015. Epub 2008 May 21. J Leukoc Biol. 2008. PMID: 18495787 Free PMC article.
-
Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via cryopyrin/NLRP3 independently of Nod2.J Immunol. 2008 Mar 15;180(6):4050-7. doi: 10.4049/jimmunol.180.6.4050. J Immunol. 2008. PMID: 18322214
-
Inflammasomes: guardians of cytosolic sanctity.Immunol Rev. 2009 Jan;227(1):95-105. doi: 10.1111/j.1600-065X.2008.00730.x. Immunol Rev. 2009. PMID: 19120479 Review.
-
Anthrax and the inflammasome.Microbes Infect. 2012 May;14(5):392-400. doi: 10.1016/j.micinf.2011.12.005. Epub 2011 Dec 17. Microbes Infect. 2012. PMID: 22207185 Free PMC article. Review.
Cited by
-
Expression analysis of inflammasomes in experimental models of inflammatory and fibrotic liver disease.J Inflamm (Lond). 2012 Nov 28;9(1):49. doi: 10.1186/1476-9255-9-49. J Inflamm (Lond). 2012. PMID: 23192004 Free PMC article.
-
Alarmins, inflammasomes and immunity.Biomed J. 2012 Nov-Dec;35(6):437-49. doi: 10.4103/2319-4170.104408. Biomed J. 2012. PMID: 23442356 Free PMC article. Review.
-
Mycobacterium tuberculosis infection of dendritic cells leads to partially caspase-1/11-independent IL-1β and IL-18 secretion but not to pyroptosis.PLoS One. 2012;7(7):e40722. doi: 10.1371/journal.pone.0040722. Epub 2012 Jul 24. PLoS One. 2012. PMID: 22911706 Free PMC article.
-
Effector functions of NLRs in the intestine: innate sensing, cell death, and disease.Immunol Res. 2012 Dec;54(1-3):25-36. doi: 10.1007/s12026-012-8317-3. Immunol Res. 2012. PMID: 22454103 Review.
-
Interplay between Peptidoglycan Biology and Virulence in Gram-Negative Pathogens.Microbiol Mol Biol Rev. 2018 Sep 12;82(4):e00033-18. doi: 10.1128/MMBR.00033-18. Print 2018 Dec. Microbiol Mol Biol Rev. 2018. PMID: 30209071 Free PMC article. Review.
References
-
- Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. - PubMed
-
- Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. - PubMed
-
- Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20:S1–13. - PubMed
-
- Dinarello CA. Infection, fever, and exogenous and endogenous pyrogens: some concepts have changed. J Endotoxin Res. 2004;10:201–222. - PubMed
-
- Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

