An essential role for Frizzled5 in neuronal survival in the parafascicular nucleus of the thalamus
- PMID: 18509025
- PMCID: PMC6670808
- DOI: 10.1523/JNEUROSCI.1056-08.2008
An essential role for Frizzled5 in neuronal survival in the parafascicular nucleus of the thalamus
Abstract
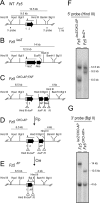
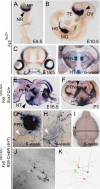
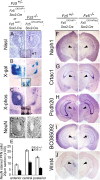
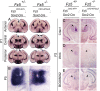
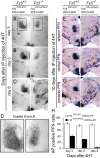
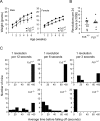
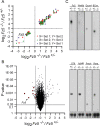
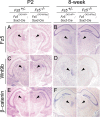
Frizzled5 (Fz5), a putative Wnt receptor, is expressed in the retina, hypothalamus, and the parafascicular nucleus (PFN) of the thalamus. By constructing Fz5 alleles in which beta-galactosidase replaces Fz5 or in which Cre-mediated recombination replaces Fz5 with alkaline phosphatase, we observe that Fz5 is required continuously and in a cell autonomous manner for the survival of adult PFN neurons, but is not required for proliferation, migration, or axonal growth and targeting of developing PFN neurons. A motor phenotype associated with loss of Fz5 establishes a role for the PFN in sensorimotor coordination. Transcripts coding for Wnt9b, the likely Fz5 ligand in vivo, and beta-catenin, a mediator of canonical Wnt signaling, are both downregulated in the Fz5(-/-) PFN, implying a positive feedback mechanism in which Wnt signaling is required to maintain the expression of Wnt signaling components. These data suggest that defects in Wnt-Frizzled signaling could be the cause of neuronal loss in degenerative CNS diseases.
Figures
Similar articles
-
Regulation of retinal progenitor expansion by Frizzled receptors: implications for microphthalmia and retinal coloboma.Hum Mol Genet. 2012 Apr 15;21(8):1848-60. doi: 10.1093/hmg/ddr616. Epub 2012 Jan 6. Hum Mol Genet. 2012. PMID: 22228100 Free PMC article.
-
Frizzled-5, a receptor for the synaptic organizer Wnt7a, regulates activity-mediated synaptogenesis.Development. 2010 Jul;137(13):2215-25. doi: 10.1242/dev.046722. Development. 2010. PMID: 20530549 Free PMC article.
-
Wnt-independent activation of beta-catenin mediated by a Dkk1-Fz5 fusion protein.Biochem Biophys Res Commun. 2005 Mar 11;328(2):533-9. doi: 10.1016/j.bbrc.2005.01.009. Biochem Biophys Res Commun. 2005. PMID: 15694380
-
Wnt/frizzled signaling during vertebrate retinal development.Dev Neurosci. 2004;26(5-6):352-8. doi: 10.1159/000082277. Dev Neurosci. 2004. PMID: 15855764 Review.
-
Impact of WNT signaling on tissue lineage differentiation in the early mouse embryo.Dev Growth Differ. 2011 Sep;53(7):843-56. doi: 10.1111/j.1440-169X.2011.01292.x. Epub 2011 Jul 15. Dev Growth Differ. 2011. PMID: 21762130 Review.
Cited by
-
Patterning and compartment formation in the diencephalon.Front Neurosci. 2012 May 11;6:66. doi: 10.3389/fnins.2012.00066. eCollection 2012. Front Neurosci. 2012. PMID: 22593732 Free PMC article.
-
Genetically-directed, cell type-specific sparse labeling for the analysis of neuronal morphology.PLoS One. 2008;3(12):e4099. doi: 10.1371/journal.pone.0004099. Epub 2008 Dec 31. PLoS One. 2008. PMID: 19116659 Free PMC article.
-
A Comprehensive Overview of Skeletal Phenotypes Associated with Alterations in Wnt/β-catenin Signaling in Humans and Mice.Bone Res. 2013 Mar 29;1(1):27-71. doi: 10.4248/BR201301004. eCollection 2013 Mar. Bone Res. 2013. PMID: 26273492 Free PMC article. Review.
-
Distinct roles of transcription factors brn3a and brn3b in controlling the development, morphology, and function of retinal ganglion cells.Neuron. 2009 Mar 26;61(6):852-64. doi: 10.1016/j.neuron.2009.01.020. Neuron. 2009. PMID: 19323995 Free PMC article.
-
Research resource: Gene profiling of G protein-coupled receptors in the arcuate nucleus of the female.Mol Endocrinol. 2014 Aug;28(8):1362-80. doi: 10.1210/me.2014-1103. Epub 2014 Jun 16. Mol Endocrinol. 2014. PMID: 24933249 Free PMC article.
References
-
- Aymerich MS, Barroso-Chinea P, Perez-Manso M, Munoz-Patino AM, Moreno-Igoa M, Gonzalez-Hernandez T, Lanciego JL. Consequences of unilateral nigro-striatal denervation on the thalamostriatal pathway in rats. Eur J Neurosci. 2006;23:2099–2108. - PubMed
-
- Backman CM, Zhang Y, Hoffer BJ, Tomac AC. Tetracycline-inducible expression systems for the generation of transgenic animals: a comparison of various inducible systems carried in a single vector. J Neurosci Methods. 2004;139:257–262. - PubMed
-
- Carmichael J, Sugars KL, Bao YP, Rubinsztein DC. Glycogen synthase kinase-3β inhibitors prevent cellular polyglutamine toxicity caused by the Huntington's disease mutation. J Biol Chem. 2002;277:33791–33798. - PubMed
-
- Carr KD, Bak TH. Medial thalamic injection of opioid agonists: mu-agonist increases while kappa-agonist decreases stimulus thresholds for pain and reward. Brain Res. 1988;441:173–184. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
