Antibodies to potato virus Y bind the amyloid beta peptide: immunohistochemical and NMR studies
- PMID: 18505725
- PMCID: PMC2504870
- DOI: 10.1074/jbc.M802088200
Antibodies to potato virus Y bind the amyloid beta peptide: immunohistochemical and NMR studies
Abstract
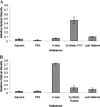
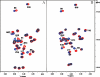
Studies in transgenic mice bearing mutated human Alzheimer disease (AD) genes show that active vaccination with the amyloid beta (Abeta) protein or passive immunization with anti-Abeta antibodies has beneficial effects on the development of disease. Although a trial of Abeta vaccination in humans was halted because of autoimmune meningoencephalitis, favorable effects on Abeta deposition in the brain and on behavior were seen. Conflicting results have been observed concerning the relationship of circulating anti-Abeta antibodies and AD. Although these autoantibodies are thought to arise from exposure to Abeta, it is also possible that homologous proteins may induce antibody synthesis. We propose that the long-standing presence of anti-Abeta antibodies or antibodies to immunogens homologous to the Abeta protein may produce protective effects. The amino acid sequence of the potato virus Y (PVY) nuclear inclusion b protein is highly homologous to the immunogenic N-terminal region of Abeta. PVY infects potatoes and related crops worldwide. Here, we show through immunocytochemistry, enzyme-linked immunosorbent assay, and NMR studies that mice inoculated with PVY develop antibodies that bind to Abeta in both neuritic plaques and neurofibrillary tangles, whereas antibodies to material from uninfected potato leaf show only modest levels of background immunoreactivity. NMR data show that the anti-PVY antibody binds to Abeta within the Phe4-Ser8 and His13-Leu17 regions. Immune responses generated from dietary exposure to proteins homologous to Abeta may induce antibodies that could influence the normal physiological processing of the protein and the development or progression of AD.
Figures
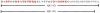

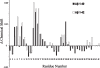
Similar articles
-
A cyclic undecamer peptide mimics a turn in folded Alzheimer amyloid β and elicits antibodies against oligomeric and fibrillar amyloid and plaques.PLoS One. 2011 Apr 19;6(4):e19110. doi: 10.1371/journal.pone.0019110. PLoS One. 2011. PMID: 21526148 Free PMC article.
-
Active full-length DNA Aβ42 immunization in 3xTg-AD mice reduces not only amyloid deposition but also tau pathology.Alzheimers Res Ther. 2018 Nov 20;10(1):115. doi: 10.1186/s13195-018-0441-4. Alzheimers Res Ther. 2018. PMID: 30454039 Free PMC article.
-
Diversity of senile plaques in Alzheimer's disease as revealed by a new monoclonal antibody that recognizes an internal sequence of the Abeta peptide.Curr Alzheimer Res. 2005 Oct;2(4):409-17. doi: 10.2174/156720505774330500. Curr Alzheimer Res. 2005. PMID: 16248846
-
Novel Abeta immunogens: is shorter better?Curr Alzheimer Res. 2007 Sep;4(4):427-36. doi: 10.2174/156720507781788800. Curr Alzheimer Res. 2007. PMID: 17908047 Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
Neuroprotective natural antibodies to assemblies of amyloidogenic peptides decrease with normal aging and advancing Alzheimer's disease.Proc Natl Acad Sci U S A. 2009 Jul 21;106(29):12145-50. doi: 10.1073/pnas.0904866106. Epub 2009 Jul 6. Proc Natl Acad Sci U S A. 2009. PMID: 19581601 Free PMC article.
-
Aquaporin 4 molecular mimicry and implications for neuromyelitis optica.J Neuroimmunol. 2013 Jul 15;260(1-2):92-8. doi: 10.1016/j.jneuroim.2013.04.015. Epub 2013 May 9. J Neuroimmunol. 2013. PMID: 23664693 Free PMC article.
-
Humans have antibodies against a plant virus: evidence from tobacco mosaic virus.PLoS One. 2013;8(4):e60621. doi: 10.1371/journal.pone.0060621. Epub 2013 Apr 3. PLoS One. 2013. PMID: 23573274 Free PMC article.
-
Recombinant helical plant virus-based nanoparticles for vaccination and immunotherapy.Virus Genes. 2018 Oct;54(5):623-637. doi: 10.1007/s11262-018-1583-y. Epub 2018 Jul 14. Virus Genes. 2018. PMID: 30008053 Review.
-
Lysine-targeting inhibition of amyloid β oligomerization by a green perilla-derived metastable chalcone in vitro and in vivo.RSC Chem Biol. 2022 Oct 18;3(12):1380-1396. doi: 10.1039/d2cb00194b. eCollection 2022 Nov 30. RSC Chem Biol. 2022. PMID: 36544574 Free PMC article.
References
-
- Rogaeva, E., Kawarai, T., and St George-Hyslop, P. (2006) J. Alzheimer's Dis. 9 381-387 - PubMed
-
- Myers, R. H., Schaefer, E. J., Wilson, P. W., D'Agostino, R., Ordovas, J. M., Espino, A., Au, R., White, R. F., Knoefel, J. E., Cobb, J. L., McNulty, K. A., Beiser, A., and Wolf, P. A. (1996) Neurology 46 673-677 - PubMed
-
- Schenk, D., Barbour, R., Dunn, W., Gordon, G., Grajeda, H., Guido, T., Hu, K., Huang, J., Johnson-Wood, K., Khan, K., Kholodenko, D., Lee, M., Liao, Z., Lieberburg, I., Motter, R., Mutter, L., Soriano, F., Shopp, G., Vasquez, N., Vandevert, C., Walker, S., Wogulis, M., Yednock, T., Games, D., and Seubert, P. (1999) Nature 400 173-177 - PubMed
-
- Schenk, D. B., Seubert, P., Grundman, M., and Black, R. (2005) Neurodegener. Dis. 2 255-260 - PubMed
-
- Furlan, R., Brambilla, E., Sanvito, F., Roccatagliata, L., Olivieri, S., Bergami, A., Pluchino, S., Uccelli, A., Comi, G., and Martino, G. (2003) Brain 126 285-291 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

