Cell-surface nucleolin is a signal transducing P-selectin binding protein for human colon carcinoma cells
- PMID: 18504038
- PMCID: PMC2504360
- DOI: 10.1016/j.yexcr.2008.03.016
Cell-surface nucleolin is a signal transducing P-selectin binding protein for human colon carcinoma cells
Abstract
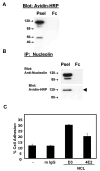
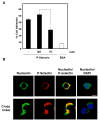
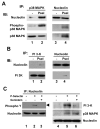
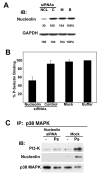
We have previously shown that P-selectin binding to Colo-320 human colon carcinoma cells induces specific activation of the alpha(5)beta(1) integrin with a concomitant increase of cell adhesion and spreading on fibronectin substrates in a phosphatidylinositol 3-kinase (PI3-K) and p38 MAPK-dependent manner. Here, we identified by affinity chromatography and characterized nucleolin as a P-selectin receptor on Colo-320 cells. Nucleolin mAb D3 significantly decreases the Colo-320 cell adhesion to immobilized P-selectin-IgG-Fc. Moreover, nucleolin becomes clustered at the external side of the plasma membrane of living, intact cells when bound to cross-linked P-selectin-IgG-Fc chimeric protein. We have also found P-selectin binding to Colo-320 cells induces tyrosine phosphorylation specifically of cell-surface nucleolin and formation of a signaling complex containing cell-surface nucleolin, PI3-K and p38 MAPK. Using siRNA approaches, we have found that both P-selectin binding to Colo-320 cells and formation of the P-selectin-mediated p38 MAPK/PI3-K signaling complex require nucleolin expression. These results show that nucleolin (or a nucleolin-like protein) is a signaling receptor for P-selectin on Colo-320 cells and suggest a mechanism for linkage of nucleolin to P-selectin-induced signal transduction pathways that regulate the adhesion and the spreading of Colo-320 on fibronectin substrates.
Figures
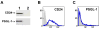

Similar articles
-
P-selectin activates integrin-mediated colon carcinoma cell adhesion to fibronectin.Exp Cell Res. 2006 Dec 10;312(20):4056-69. doi: 10.1016/j.yexcr.2006.09.008. Epub 2006 Sep 16. Exp Cell Res. 2006. PMID: 17056038 Free PMC article.
-
Hsa-miR-370 inhibited P-selectin-induced cell adhesion in human colon adenocarcinoma cells.Mol Cell Biochem. 2019 Jan;450(1-2):159-166. doi: 10.1007/s11010-018-3382-0. Epub 2018 Jun 19. Mol Cell Biochem. 2019. PMID: 29922946
-
Nucleophosmin and nucleolin regulate K-Ras plasma membrane interactions and MAPK signal transduction.J Biol Chem. 2009 Oct 9;284(41):28410-28419. doi: 10.1074/jbc.M109.001537. Epub 2009 Aug 6. J Biol Chem. 2009. PMID: 19661056 Free PMC article.
-
Cloning and functional characterization of recombinant equine P-selectin.Vet Immunol Immunopathol. 2007 Apr 15;116(3-4):115-30. doi: 10.1016/j.vetimm.2007.01.004. Epub 2007 Jan 16. Vet Immunol Immunopathol. 2007. PMID: 17306378
-
Midkine, a cytokine that inhibits HIV infection by binding to the cell surface expressed nucleolin.Cell Res. 2006 Feb;16(2):174-81. doi: 10.1038/sj.cr.7310024. Cell Res. 2006. PMID: 16474431 Review.
Cited by
-
Surface expressed nucleolin is constantly induced in tumor cells to mediate calcium-dependent ligand internalization.PLoS One. 2010 Dec 23;5(12):e15787. doi: 10.1371/journal.pone.0015787. PLoS One. 2010. PMID: 21203423 Free PMC article.
-
Identification of nucleolin as a cellular receptor for human respiratory syncytial virus.Nat Med. 2011 Aug 14;17(9):1132-5. doi: 10.1038/nm.2444. Nat Med. 2011. PMID: 21841784
-
The lectin-binding pattern of nucleolin and its interaction with endogenous galectin-3.Cell Mol Biol Lett. 2014 Sep;19(3):461-82. doi: 10.2478/s11658-014-0206-4. Epub 2014 Aug 29. Cell Mol Biol Lett. 2014. PMID: 25169435 Free PMC article.
-
Cancer Stem Cells and Nucleolin as Drivers of Carcinogenesis.Pharmaceuticals (Basel). 2021 Jan 13;14(1):60. doi: 10.3390/ph14010060. Pharmaceuticals (Basel). 2021. PMID: 33451077 Free PMC article. Review.
-
RNA-binding protein nucleolin in disease.RNA Biol. 2012 Jun;9(6):799-808. doi: 10.4161/rna.19718. Epub 2012 May 23. RNA Biol. 2012. PMID: 22617883 Free PMC article. Review.
References
-
- Gassmann P, Enns A, Haier J. Role of tumor cell adhesion and migration in organ-specific metastasis formation. Onkologie. 2004;27:577–582. - PubMed
-
- Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–458. - PubMed
-
- MacDonald I, Groom A, Chambers A. Cancer spread and micrometastasis development: quantitative approaches for in vivo models. Bioessays. 2002;24:885–893. - PubMed
-
- Haier J, Nicolson G. Tumor cell adhesion under hydrodynamic conditions of fluid flow. APMIS. 2001;109:241–262. - PubMed
-
- Dejana E, Martin-Padura D, Lauri S, Bernasconi M, Bani A. Endothelial leukocyte adhesion molecule-1-dependent adhesion of colon carcinoma cells to vascular endothelium is inhibited by an anti-body to Lewis fucosylated type I carbohydrate chain. Lab Invest. 1992;66:324–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

