Molecular mechanism and structure of Trigger Factor bound to the translating ribosome
- PMID: 18497744
- PMCID: PMC2426727
- DOI: 10.1038/emboj.2008.89
Molecular mechanism and structure of Trigger Factor bound to the translating ribosome
Abstract
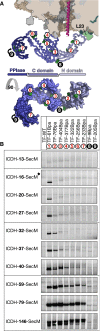
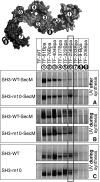
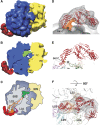
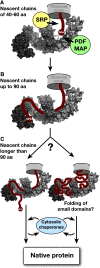
Ribosome-associated chaperone Trigger Factor (TF) initiates folding of newly synthesized proteins in bacteria. Here, we pinpoint by site-specific crosslinking the sequence of molecular interactions of Escherichia coli TF and nascent chains during translation. Furthermore, we provide the first full-length structure of TF associated with ribosome-nascent chain complexes by using cryo-electron microscopy. In its active state, TF arches over the ribosomal exit tunnel accepting nascent chains in a protective void. The growing nascent chain initially follows a predefined path through the entire interior of TF in an unfolded conformation, and even after folding into a domain it remains accommodated inside the protective cavity of ribosome-bound TF. The adaptability to accept nascent chains of different length and folding states may explain how TF is able to assist co-translational folding of all kinds of nascent polypeptides during ongoing synthesis. Moreover, we suggest a model of how TF's chaperoning function can be coordinated with the co-translational processing and membrane targeting of nascent polypeptides by other ribosome-associated factors.
Figures



Similar articles
-
A cradle for new proteins: trigger factor at the ribosome.Curr Opin Struct Biol. 2005 Apr;15(2):204-12. doi: 10.1016/j.sbi.2005.03.005. Curr Opin Struct Biol. 2005. PMID: 15837180 Review.
-
Dynamic Behavior of Trigger Factor on the Ribosome.J Mol Biol. 2016 Sep 11;428(18):3588-602. doi: 10.1016/j.jmb.2016.06.007. Epub 2016 Jun 16. J Mol Biol. 2016. PMID: 27320387
-
Real-time observation of trigger factor function on translating ribosomes.Nature. 2006 Nov 23;444(7118):455-60. doi: 10.1038/nature05225. Epub 2006 Oct 15. Nature. 2006. PMID: 17051157
-
L23 protein functions as a chaperone docking site on the ribosome.Nature. 2002 Sep 12;419(6903):171-4. doi: 10.1038/nature01047. Nature. 2002. PMID: 12226666
-
The ribosome as a platform for co-translational processing, folding and targeting of newly synthesized proteins.Nat Struct Mol Biol. 2009 Jun;16(6):589-97. doi: 10.1038/nsmb.1614. Nat Struct Mol Biol. 2009. PMID: 19491936 Review.
Cited by
-
Protein export through the bacterial Sec pathway.Nat Rev Microbiol. 2017 Jan;15(1):21-36. doi: 10.1038/nrmicro.2016.161. Epub 2016 Nov 28. Nat Rev Microbiol. 2017. PMID: 27890920 Review.
-
A protean clamp guides membrane targeting of tail-anchored proteins.Proc Natl Acad Sci U S A. 2017 Oct 10;114(41):E8585-E8594. doi: 10.1073/pnas.1708731114. Epub 2017 Sep 26. Proc Natl Acad Sci U S A. 2017. PMID: 28973888 Free PMC article.
-
Bayesian reweighting of biomolecular structural ensembles using heterogeneous cryo-EM maps with the cryoENsemble method.Sci Rep. 2024 Aug 5;14(1):18149. doi: 10.1038/s41598-024-68468-7. Sci Rep. 2024. PMID: 39103467 Free PMC article.
-
Acceleration of protein folding by four orders of magnitude through a single amino acid substitution.Sci Rep. 2015 Jun 30;5:11840. doi: 10.1038/srep11840. Sci Rep. 2015. PMID: 26121966 Free PMC article.
-
The molecular timeline of a reviving bacterial spore.Mol Cell. 2015 Feb 19;57(4):695-707. doi: 10.1016/j.molcel.2014.12.019. Epub 2015 Feb 5. Mol Cell. 2015. PMID: 25661487 Free PMC article.
References
-
- Agashe VR, Guha S, Chang HC, Genevaux P, Hayer-Hartl M, Stemp M, Georgopoulos C, Hartl FU, Barral JM (2004) Function of trigger factor and DnaK in multidomain protein folding: increase in yield at the expense of folding speed. Cell 117: 199–209 - PubMed
-
- Ball LA, Kaesberg P (1973) Cleavage of the N-terminal formylmethionine residue from a bacteriophage coat protein in vitro. J Mol Biol 79: 531–537 - PubMed
-
- Blanco FJ, Angrand I, Serrano L (1999) Exploring the conformational properties of the sequence space between two proteins with different folds: an experimental study. J Mol Biol 285: 741–753 - PubMed
-
- Bukau B, Deuerling E, Pfund C, Craig EA (2000) Getting newly synthesized proteins into shape. Cell 101: 119–122 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

