Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2
- PMID: 18495771
- PMCID: PMC2493326
- DOI: 10.1128/JVI.00737-08
Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2
Abstract
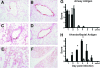
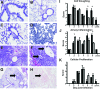
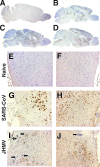
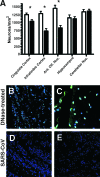
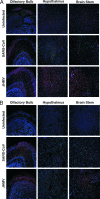
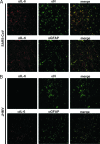
Infection of humans with the severe acute respiratory syndrome coronavirus (SARS-CoV) results in substantial morbidity and mortality, with death resulting primarily from respiratory failure. While the lungs are the major site of infection, the brain is also infected in some patients. Brain infection may result in long-term neurological sequelae, but little is known about the pathogenesis of SARS-CoV in this organ. We previously showed that the brain was a major target organ for infection in mice that are transgenic for the SARS-CoV receptor (human angiotensin-converting enzyme 2). Herein, we use these mice to show that virus enters the brain primarily via the olfactory bulb, and infection results in rapid, transneuronal spread to connected areas of the brain. This extensive neuronal infection is the main cause of death because intracranial inoculation with low doses of virus results in a uniformly lethal disease even though little infection is detected in the lungs. Death of the animal likely results from dysfunction and/or death of infected neurons, especially those located in cardiorespiratory centers in the medulla. Remarkably, the virus induces minimal cellular infiltration in the brain. Our results show that neurons are a highly susceptible target for SARS-CoV and that only the absence of the host cell receptor prevents severe murine brain disease.
Figures

Similar articles
-
Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus.J Virol. 2007 Jan;81(2):813-21. doi: 10.1128/JVI.02012-06. Epub 2006 Nov 1. J Virol. 2007. PMID: 17079315 Free PMC article.
-
Severe acute respiratory syndrome coronavirus infection of mice transgenic for the human Angiotensin-converting enzyme 2 virus receptor.J Virol. 2007 Feb;81(3):1162-73. doi: 10.1128/JVI.01702-06. Epub 2006 Nov 15. J Virol. 2007. PMID: 17108019 Free PMC article.
-
Differential virological and immunological outcome of severe acute respiratory syndrome coronavirus infection in susceptible and resistant transgenic mice expressing human angiotensin-converting enzyme 2.J Virol. 2009 Jun;83(11):5451-65. doi: 10.1128/JVI.02272-08. Epub 2009 Mar 18. J Virol. 2009. PMID: 19297479 Free PMC article.
-
[Lessons from SARS: a new potential therapy for acute respiratory distress syndrome (ARDS) with angiotensin converting enzyme 2 (ACE2)].Masui. 2008 Mar;57(3):302-10. Masui. 2008. PMID: 18340998 Review. Japanese.
-
The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice.Exp Physiol. 2008 May;93(5):543-8. doi: 10.1113/expphysiol.2007.040048. Epub 2008 Apr 10. Exp Physiol. 2008. PMID: 18448662 Free PMC article. Review.
Cited by
-
Myelin oligodendrocyte glycoprotein-associated transverse myelitis after SARS-CoV-2 infection: A case report.World J Radiol. 2024 Sep 28;16(9):446-452. doi: 10.4329/wjr.v16.i9.446. World J Radiol. 2024. PMID: 39355395 Free PMC article.
-
Bilateral Perivascular Chorioretinal Atrophy Resembling Pigmented Paravenous Chorioretinal Atrophy Post COVID-19 Infection: A Case Report and Comprehensive Immune Profiling.Vaccines (Basel). 2024 Aug 2;12(8):878. doi: 10.3390/vaccines12080878. Vaccines (Basel). 2024. PMID: 39204003 Free PMC article.
-
Ocular Implications of COVID-19 Infection and Vaccine-Related Adverse Events.J Pers Med. 2024 Jul 23;14(8):780. doi: 10.3390/jpm14080780. J Pers Med. 2024. PMID: 39201972 Free PMC article. Review.
-
Mechanisms by Which SARS-CoV-2 Invades and Damages the Central Nervous System: Apart from the Immune Response and Inflammatory Storm, What Else Do We Know?Viruses. 2024 Apr 24;16(5):663. doi: 10.3390/v16050663. Viruses. 2024. PMID: 38793545 Free PMC article. Review.
-
Olfactory Dysfunction in COVID-19 Patients in a Tertiary Care Hospital; A Cross-Sectional Observational Study.Iran J Otorhinolaryngol. 2024 May;36(3):459-466. doi: 10.22038/IJORL.2024.76275.3557. Iran J Otorhinolaryngol. 2024. PMID: 38745684 Free PMC article.
References
-
- Allan, S. M., and N. J. Rothwell. 2001. Cytokines and acute neurodegeneration. Nat. Rev. Neurosci. 2734-744. - PubMed
-
- Aloisi, F. 2001. Immune function of microglia. Glia 36165-179. - PubMed
-
- Astic, L., D. Saucier, P. Coulon, F. Lafay, and A. Flamand. 1993. The CVS strain of rabies virus as transneuronal tracer in the olfactory system of mice. Brain Res. 619146-156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

