An intracellular motif of GLUT4 regulates fusion of GLUT4-containing vesicles
- PMID: 18492238
- PMCID: PMC2405794
- DOI: 10.1186/1471-2121-9-25
An intracellular motif of GLUT4 regulates fusion of GLUT4-containing vesicles
Abstract
Background: Insulin stimulates glucose uptake by adipocytes through increasing translocation of the glucose transporter GLUT4 from an intracellular compartment to the plasma membrane. Fusion of GLUT4-containing vesicles at the cell surface is thought to involve phospholipase D activity, generating the signalling lipid phosphatidic acid, although the mechanism of action is not yet clear.
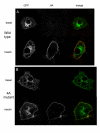
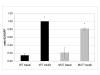
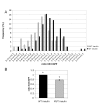
Results: Here we report the identification of a putative phosphatidic acid-binding motif in a GLUT4 intracellular loop. Mutation of this motif causes a decrease in the insulin-induced exposure of GLUT4 at the cell surface of 3T3-L1 adipocytes via an effect on vesicle fusion.
Conclusion: The potential phosphatidic acid-binding motif identified in this study is unique to GLUT4 among the sugar transporters, therefore this motif may provide a unique mechanism for regulating insulin-induced translocation by phospholipase D signalling.
Figures

Similar articles
-
Identification of amino acid residues within the C terminus of the Glut4 glucose transporter that are essential for insulin-stimulated redistribution to the plasma membrane.J Biol Chem. 2008 May 2;283(18):12571-85. doi: 10.1074/jbc.M800838200. Epub 2008 Feb 27. J Biol Chem. 2008. PMID: 18305115 Free PMC article.
-
The glucose transporter 4 FQQI motif is necessary for Akt substrate of 160-kilodalton-dependent plasma membrane translocation but not Golgi-localized (gamma)-ear-containing Arf-binding protein-dependent entry into the insulin-responsive storage compartment.Mol Endocrinol. 2007 Dec;21(12):3087-99. doi: 10.1210/me.2006-0476. Epub 2007 Aug 30. Mol Endocrinol. 2007. PMID: 17761952
-
Single point mutations result in the miss-sorting of Glut4 to a novel membrane compartment associated with stress granule proteins.PLoS One. 2013 Jul 16;8(7):e68516. doi: 10.1371/journal.pone.0068516. Print 2013. PLoS One. 2013. PMID: 23874650 Free PMC article.
-
GLUT4 translocation: the last 200 nanometers.Cell Signal. 2007 Nov;19(11):2209-17. doi: 10.1016/j.cellsig.2007.06.003. Epub 2007 Jun 21. Cell Signal. 2007. PMID: 17629673 Review.
-
GLUT4 dispersal at the plasma membrane of adipocytes: a super-resolved journey.Biosci Rep. 2023 Oct 31;43(10):BSR20230946. doi: 10.1042/BSR20230946. Biosci Rep. 2023. PMID: 37791639 Free PMC article. Review.
Cited by
-
Synaptic vesicle-like lipidome of human cytomegalovirus virions reveals a role for SNARE machinery in virion egress.Proc Natl Acad Sci U S A. 2011 Aug 2;108(31):12869-74. doi: 10.1073/pnas.1109796108. Epub 2011 Jul 18. Proc Natl Acad Sci U S A. 2011. PMID: 21768361 Free PMC article.
-
Potential role of sugar transporters in cancer and their relationship with anticancer therapy.Int J Endocrinol. 2010;2010:205357. doi: 10.1155/2010/205357. Epub 2010 Jul 18. Int J Endocrinol. 2010. PMID: 20706540 Free PMC article.
References
-
- Iyer SS, Barton JA, Bourgoin S, Kusner DJ. Phospholipases D1 and D2 coordinately regulate macrophage phagocytosis. J Immunol. 2004;173:2615–2623. - PubMed
-
- Baillie GS, Huston E, Scotland G, Hodgkin M, Gall I, Peden AH, MacKenzie C, Houslay ES, Currie R, Pettitt TR, Walmsley AR, Wakelam MJ, Warwicker J, Houslay MD. TAPAS-1, a novel microdomain within the unique N-terminal region of the PDE4A1 cAMP-specific phosphodiesterase that allows rapid, Ca2+-triggered membrane association with selectivity for interaction with phosphatidic acid. J Biol Chem. 2002;277:28298–28309. doi: 10.1074/jbc.M108353200. - DOI - PubMed
-
- Ghosh S, Strum JC, Sciorra VA, Daniel L, Bell RM. Raf-1 kinase possesses distinct binding domains for phosphatidylserine and phosphatidic acid. Phosphatidic acid regulates the translocation of Raf-1 in 12-O-tetradecanoylphorbol-13-acetate-stimulated Madin-Darby canine kidney cells. J Biol Chem. 1996;271:8472–8480. doi: 10.1074/jbc.271.14.8472. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

