Stat3 promotes metastatic progression of prostate cancer
- PMID: 18483213
- PMCID: PMC2408430
- DOI: 10.2353/ajpath.2008.071054
Stat3 promotes metastatic progression of prostate cancer
Abstract
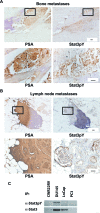
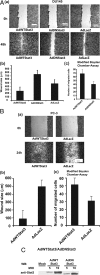
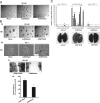
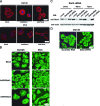
There are currently no effective therapies for metastatic prostate cancer because the molecular mechanisms that underlie the metastatic spread of primary prostate cancer are unclear. Transcription factor Stat3 is constitutively active in malignant prostate epithelium, and its activation is associated with high histological grade and advanced cancer stage. In this work, we hypothesized that Stat3 stimulates metastatic progression of prostate cancer. We show that Stat3 is active in 77% of lymph node and 67% of bone metastases of clinical human prostate cancers. Importantly, adenoviral gene delivery of wild-type Stat3 (AdWTStat3) to DU145 human prostate cancer cells increased the number of lung metastases by 33-fold in an experimental metastasis assay compared with controls. Using various methods to inhibit Stat3, we demonstrated that Stat3 promotes human prostate cancer cell migration. Stat3 induced the formation of lamellipodia in both DU145 and PC-3 cells, further supporting the concept that Stat3 promotes a migratory phenotype of human prostate cancer cells. Moreover, Stat3 caused the rearrangement of cytoplasmic actin stress fibers and microtubules in both DU145 and PC-3 cells. Finally, inhibition of the Jak2 tyrosine kinase decreased both activation of Stat3 and prostate cancer cell motility. Collectively, these data indicate that transcription factor Stat3 is involved in metastatic behavior of human prostate cancer cells and may provide a therapeutic target to prevent metastatic spread of primary prostate cancer.
Figures

Comment in
-
Vitamin D may reduce prostate cancer metastasis by several mechanisms including blocking Stat3.Am J Pathol. 2008 Nov;173(5):1589-90. doi: 10.2353/ajpath.2008.080579. Am J Pathol. 2008. PMID: 18948436 Free PMC article.
Similar articles
-
The STAT3 Inhibitor Galiellalactone Effectively Reduces Tumor Growth and Metastatic Spread in an Orthotopic Xenograft Mouse Model of Prostate Cancer.Eur Urol. 2016 Mar;69(3):400-4. doi: 10.1016/j.eururo.2015.06.016. Epub 2015 Jul 2. Eur Urol. 2016. PMID: 26144873
-
Pharmacologic suppression of JAK1/2 by JAK1/2 inhibitor AZD1480 potently inhibits IL-6-induced experimental prostate cancer metastases formation.Mol Cancer Ther. 2014 May;13(5):1246-58. doi: 10.1158/1535-7163.MCT-13-0605. Epub 2014 Feb 27. Mol Cancer Ther. 2014. PMID: 24577942 Free PMC article.
-
Inhibition of STAT3 prevents bone metastatic progression of prostate cancer in vivo.Prostate. 2021 Jun;81(8):452-462. doi: 10.1002/pros.24125. Epub 2021 Apr 6. Prostate. 2021. PMID: 33822400
-
Autocrine prolactin promotes prostate cancer cell growth via Janus kinase-2-signal transducer and activator of transcription-5a/b signaling pathway.Endocrinology. 2007 Jul;148(7):3089-101. doi: 10.1210/en.2006-1761. Epub 2007 Apr 5. Endocrinology. 2007. PMID: 17412813
-
STAT3 signaling in prostate cancer progression and therapy resistance: An oncogenic pathway with diverse functions.Biomed Pharmacother. 2023 Feb;158:114168. doi: 10.1016/j.biopha.2022.114168. Epub 2023 Jan 3. Biomed Pharmacother. 2023. PMID: 36916439 Review.
Cited by
-
Capsazepine inhibits JAK/STAT3 signaling, tumor growth, and cell survival in prostate cancer.Oncotarget. 2017 Mar 14;8(11):17700-17711. doi: 10.18632/oncotarget.10775. Oncotarget. 2017. PMID: 27458171 Free PMC article.
-
Constructing higher-order miRNA-mRNA interaction networks in prostate cancer via hypergraph-based learning.BMC Syst Biol. 2013 Jun 19;7:47. doi: 10.1186/1752-0509-7-47. BMC Syst Biol. 2013. PMID: 23782521 Free PMC article.
-
Phospholipase PLA2G7, associated with aggressive prostate cancer, promotes prostate cancer cell migration and invasion and is inhibited by statins.Oncotarget. 2011 Dec;2(12):1176-90. doi: 10.18632/oncotarget.397. Oncotarget. 2011. PMID: 22202492 Free PMC article.
-
EGCG enhances the therapeutic potential of gemcitabine and CP690550 by inhibiting STAT3 signaling pathway in human pancreatic cancer.PLoS One. 2012;7(2):e31067. doi: 10.1371/journal.pone.0031067. Epub 2012 Feb 13. PLoS One. 2012. PMID: 22348037 Free PMC article.
-
Human prostatic acid phosphatase, an authentic tyrosine phosphatase, dephosphorylates ErbB-2 and regulates prostate cancer cell growth.J Biol Chem. 2010 Jul 30;285(31):23598-606. doi: 10.1074/jbc.M109.098301. Epub 2010 May 24. J Biol Chem. 2010. PMID: 20498373 Free PMC article.
References
-
- Arya M, Bott SR, Shergill IS, Ahmed HU, Williamson M, Patel HR. The metastatic cascade in prostate cancer. Surg Oncol. 2006;15:117–128. - PubMed
-
- Ihle JN. The Stat family in cytokine signaling. Curr Opin Cell Biol. 2001;13:211–217. - PubMed
-
- Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. - PubMed
-
- Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. - PubMed
-
- Mora LB, Buettner R, Seigne J, Diaz J, Ahmad N, Garcia R, Bowman T, Falcone R, Fairclough R, Cantor A, Muro-Cacho C, Livingston S, Karras J, Pow-Sang J, Jove R. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002;62:6659–6666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

