Nephrin binds to the COOH terminus of a large-conductance Ca2+-activated K+ channel isoform and regulates its expression on the cell surface
- PMID: 18480178
- PMCID: PMC2494500
- DOI: 10.1152/ajprenal.00140.2008
Nephrin binds to the COOH terminus of a large-conductance Ca2+-activated K+ channel isoform and regulates its expression on the cell surface
Abstract
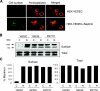
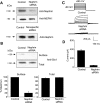
We carried out a yeast two-hybrid screen to identify proteins that interact with large-conductance Ca2+-activated K+ (BKCa) channels encoded by the Slo1 gene. Nephrin, an essential adhesion and scaffolding molecule expressed in podocytes, emerged in this screen. The Slo1-nephrin interaction was confirmed by coimmunoprecipitation from the brain and kidney, from HEK-293T cells expressing both proteins, and by glutathione S-transferase pull-down assays. We detected nephrin binding to the Slo1 VEDEC splice variant, which is typically retained in intracellular stores, and to the beta4-subunit. However, we did not detect significant binding of nephrin to the Slo1 QEERL or Slo1 EMVYR splice variants. Coexpression of nephrin with Slo1 VEDEC increased expression of functional BKCa channels on the surface of HEK-293T cells but did not affect steady-state surface expression of the other COOH-terminal Slo1 variants. Nephrin did not affect the kinetics or voltage dependence of channel activation in HEK-293T cells expressing Slo1. Stimulation of Slo1 VEDEC surface expression in HEK-293T cells was also observed by coexpressing a small construct encoding only the distal COOH-terminal domains of nephrin that interact with Slo1. Reduction of endogenous nephrin expression by application of small interfering RNA to differentiated cells of an immortalized podocyte cell line markedly reduced the steady-state surface expression of Slo1 as assessed by electrophysiology and cell-surface biotinylation assays. Nephrin therefore plays a role in organizing the surface expression of ion channel proteins in podocytes and may play a role in outside-in signaling to allow podocytes to adapt to mechanical or neurohumoral stimuli originating in neighboring cells.
Figures






Similar articles
-
Neph1 regulates steady-state surface expression of Slo1 Ca(2+)-activated K(+) channels: different effects in embryonic neurons and podocytes.Am J Physiol Cell Physiol. 2009 Dec;297(6):C1379-88. doi: 10.1152/ajpcell.00354.2009. Epub 2009 Sep 30. Am J Physiol Cell Physiol. 2009. PMID: 19794150 Free PMC article.
-
MAGI-1 interacts with Slo1 channel proteins and suppresses Slo1 expression on the cell surface.Am J Physiol Cell Physiol. 2009 Jul;297(1):C55-65. doi: 10.1152/ajpcell.00073.2009. Epub 2009 Apr 29. Am J Physiol Cell Physiol. 2009. PMID: 19403801 Free PMC article.
-
Canonical transient receptor potential channel (TRPC)3 and TRPC6 associate with large-conductance Ca2+-activated K+ (BKCa) channels: role in BKCa trafficking to the surface of cultured podocytes.Mol Pharmacol. 2009 Mar;75(3):466-77. doi: 10.1124/mol.108.051912. Epub 2008 Dec 3. Mol Pharmacol. 2009. PMID: 19052171 Free PMC article.
-
Mechanistic insight into the heme-independent interplay between iron and carbon monoxide in CFTR and Slo1 BKCa channels.Metallomics. 2017 Jun 1;9(6):634-645. doi: 10.1039/c7mt00065k. Epub 2017 May 5. Metallomics. 2017. PMID: 28474046 Free PMC article. Review.
-
Transduction of voltage and Ca2+ signals by Slo1 BK channels.Physiology (Bethesda). 2013 May;28(3):172-89. doi: 10.1152/physiol.00055.2012. Physiology (Bethesda). 2013. PMID: 23636263 Free PMC article. Review.
Cited by
-
20-Hydroxyeicosatetraenoic Acid (20-HETE) Modulates Canonical Transient Receptor Potential-6 (TRPC6) Channels in Podocytes.Front Physiol. 2016 Aug 31;7:351. doi: 10.3389/fphys.2016.00351. eCollection 2016. Front Physiol. 2016. PMID: 27630573 Free PMC article.
-
Podocytes … What's Under Yours? (Podocytes and Foot Processes and How They Change in Nephropathy).Front Endocrinol (Lausanne). 2015 Feb 23;6:9. doi: 10.3389/fendo.2015.00009. eCollection 2015. Front Endocrinol (Lausanne). 2015. PMID: 25755650 Free PMC article. Review.
-
Sustained activation of N-methyl-D-aspartate receptors in podoctyes leads to oxidative stress, mobilization of transient receptor potential canonical 6 channels, nuclear factor of activated T cells activation, and apoptotic cell death.Mol Pharmacol. 2012 Oct;82(4):728-37. doi: 10.1124/mol.112.079376. Epub 2012 Jul 24. Mol Pharmacol. 2012. PMID: 22828802 Free PMC article.
-
The beta1 subunit of Na+/K+-ATPase interacts with BKCa channels and affects their steady-state expression on the cell surface.FEBS Lett. 2009 Oct 6;583(19):3109-14. doi: 10.1016/j.febslet.2009.08.039. Epub 2009 Sep 1. FEBS Lett. 2009. PMID: 19729011 Free PMC article.
-
Identification and quantification of full-length BK channel variants in the developing mouse cochlea.J Neurosci Res. 2011 Nov;89(11):1747-60. doi: 10.1002/jnr.22713. Epub 2011 Jul 28. J Neurosci Res. 2011. PMID: 21800349 Free PMC article.
References
-
- Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel β-subunits, hKCNMB3 and hKCNMB4. J Biol Chem 275: 6453–6461, 2000. - PubMed
-
- Cameron JS, Dryer SE. BK-type KCa channels in two parasympathetic cell types: differences in kinetic properties and developmental expression. J Neurophysiol 84: 2767–2776, 2000. - PubMed
-
- Chae KS, Martin-Caraballo M, Anderson M, Dryer SE. Akt activation is necessary for growth factor-induced trafficking of functional KCa channels in developing parasympathetic neurons. J Neurophysiol 93: 1174–1182, 2005. - PubMed
-
- Coward RJ, Welsh GI, Koziell A, Hussain S, Lennon R, Ni L, Tavaré JM, Mathieson PW, Saleem MA. Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes 56: 1127–1135, 2007. - PubMed
-
- Drenckhahn D, Franke RP. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest 59: 673–682, 1988. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

