Neural stem/progenitor cells promote endothelial cell morphogenesis and protect endothelial cells against ischemia via HIF-1alpha-regulated VEGF signaling
- PMID: 18478024
- PMCID: PMC3018350
- DOI: 10.1038/jcbfm.2008.38
Neural stem/progenitor cells promote endothelial cell morphogenesis and protect endothelial cells against ischemia via HIF-1alpha-regulated VEGF signaling
Abstract
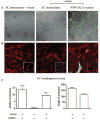
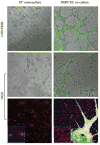
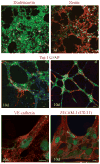
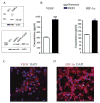
Vascular cells provide a neural stem/progenitor cell (NSPC) niche that regulates expansion and differentiation of NSPCs within the germinal zones of the embryonic and adult brain under both physiologic and pathologic conditions. Here, we examined the NSPC-endothelial cell (NSPC/EC) interaction under conditions of ischemia, both in vitro and after intracerebral transplantation. In culture, embryonic mouse NSPCs supported capillary morphogenesis and protected ECs from cell death induced by serum starvation or by transient oxygen and glucose deprivation (OGD). Neural stem/progenitor cells constitutively expressed hypoxia-inducible factor 1alpha (HIF-1alpha) transcription factor and vascular endothelial growth factor (VEGF), both of which were increased approximately twofold after the exposure of NSPCs to OGD. The protective effects of NSPCs on ECs under conditions of serum starvation and hypoxia were blocked by pharmacological inhibitors of VEGF signaling, SU1498 and Flt-1-Fc. After intracerebral transplantation, NSPCs continued to express HIF-1alpha and VEGF, and promoted microvascular density after focal ischemia. These studies support a role for NSPCs in stabilization of vasculature during ischemia, mediated via HIF-1alpha-VEGF signaling pathways, and suggest therapeutic application of NSPCs to promote revascularization and repair after brain injury.
Figures
Similar articles
-
Murine neural stem/progenitor cells protect neurons against ischemia by HIF-1alpha-regulated VEGF signaling.PLoS One. 2010 Mar 22;5(3):e9767. doi: 10.1371/journal.pone.0009767. PLoS One. 2010. PMID: 20339541 Free PMC article.
-
Catalpol protects vascular structure and promotes angiogenesis in cerebral ischemic rats by targeting HIF-1α/VEGF.Phytomedicine. 2020 Nov;78:153300. doi: 10.1016/j.phymed.2020.153300. Epub 2020 Aug 28. Phytomedicine. 2020. PMID: 32866905
-
Transplantation of human neural stem cells protect against ischemia in a preventive mode via hypoxia-inducible factor-1alpha stabilization in the host brain.Brain Res. 2008 May 1;1207:182-92. doi: 10.1016/j.brainres.2008.02.043. Epub 2008 Mar 4. Brain Res. 2008. PMID: 18371939
-
Critical role of hypoxia sensor--HIF-1α in VEGF gene activation. Implications for angiogenesis and tissue injury healing.Curr Med Chem. 2012;19(1):90-7. doi: 10.2174/092986712803413944. Curr Med Chem. 2012. PMID: 22300081 Review.
-
Roles for HIF-1α in neural stem cell function and the regenerative response to stroke.Behav Brain Res. 2012 Feb 14;227(2):410-7. doi: 10.1016/j.bbr.2011.08.002. Epub 2011 Aug 18. Behav Brain Res. 2012. PMID: 21871501 Free PMC article. Review.
Cited by
-
HIF-1 alpha-deficient mice have increased brain injury after neonatal hypoxia-ischemia.Dev Neurosci. 2009;31(5):452-8. doi: 10.1159/000232563. Epub 2009 Aug 11. Dev Neurosci. 2009. PMID: 19672073 Free PMC article.
-
Bone marrow cell cotransplantation with islets improves their vascularization and function.Transplantation. 2010 Mar 27;89(6):686-93. doi: 10.1097/TP.0b013e3181cb3e8d. Transplantation. 2010. PMID: 20101199 Free PMC article.
-
Regulation of tissue morphogenesis by endothelial cell-derived signals.Trends Cell Biol. 2015 Mar;25(3):148-57. doi: 10.1016/j.tcb.2014.11.007. Epub 2014 Dec 17. Trends Cell Biol. 2015. PMID: 25529933 Free PMC article. Review.
-
The Role of a Neurovascular Signaling Pathway Involving Hypoxia-Inducible Factor and Notch in the Function of the Central Nervous System.Biomol Ther (Seoul). 2020 Jan 1;28(1):45-57. doi: 10.4062/biomolther.2019.119. Biomol Ther (Seoul). 2020. PMID: 31484285 Free PMC article. Review.
-
Focal cerebral ischemia induces a multilineage cytogenic response from adult subventricular zone that is predominantly gliogenic.Glia. 2010 Oct;58(13):1610-9. doi: 10.1002/glia.21033. Glia. 2010. PMID: 20578055 Free PMC article.
References
-
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–6. - PubMed
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–70. - PubMed
-
- Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. Stem Cell-like Glioma Cells Promote Tumor Angiogenesis through Vascular Endothelial Growth Factor. Cancer Res. 2006;66:7843–8. - PubMed
-
- Brusselmans K, Bono F, Collen D, Herbert JM, Carmeliet P, Dewerchin M. A novel role for vascular endothelial growth factor as an autocrine survival factor for embryonic stem cells during hypoxia. J Biol Chem. 2005;280:3493–9. - PubMed
-
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

