Unique small molecule entry inhibitors of hemorrhagic fever arenaviruses
- PMID: 18474596
- PMCID: PMC2441566
- DOI: 10.1074/jbc.M802089200
Unique small molecule entry inhibitors of hemorrhagic fever arenaviruses
Abstract
Viral hemorrhagic fevers caused by the arenaviruses Lassa virus in Africa and Machupo, Guanarito, Junin, and Sabia virus in South America are among the most devastating emerging human diseases with fatality rates of 15-35% and a limited antiviral therapeutic repertoire available. Here we used high throughput screening of synthetic combinatorial small molecule libraries to identify inhibitors of arenavirus infection using pseudotyped virion particles bearing the glycoproteins (GPs) of highly pathogenic arenaviruses. Our screening efforts resulted in the discovery of a series of novel small molecule inhibitors of viral entry that are highly active against both Old World and New World hemorrhagic arenaviruses. We observed potent inhibition of infection of human and primate cells with live hemorrhagic arenaviruses (IC(50)=500-800 nm). Investigations of the mechanism of action revealed that the candidate compounds efficiently block pH-dependent fusion by the arenavirus GPs (IC(50) of 200-350 nm). Although our lead compounds were potent against phylogenetically distant arenaviruses, they did not show activity against other enveloped viruses with class I viral fusion proteins, indicating specificity for arenavirus GP-mediated membrane fusion.
Figures
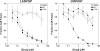

Similar articles
-
Identification of Clotrimazole Derivatives as Specific Inhibitors of Arenavirus Fusion.J Virol. 2019 Mar 5;93(6):e01744-18. doi: 10.1128/JVI.01744-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30626681 Free PMC article.
-
Drug discovery technologies and strategies for Machupo virus and other New World arenaviruses.Expert Opin Drug Discov. 2012 Jul;7(7):613-32. doi: 10.1517/17460441.2012.687719. Epub 2012 May 19. Expert Opin Drug Discov. 2012. PMID: 22607481 Free PMC article. Review.
-
Identification and characterization of potent small molecule inhibitor of hemorrhagic fever New World arenaviruses.Antiviral Res. 2006 Feb;69(2):86-97. doi: 10.1016/j.antiviral.2005.10.008. Epub 2005 Nov 28. Antiviral Res. 2006. PMID: 16343651 Free PMC article.
-
Current drug discovery strategies against arenavirus infections.Expert Rev Anti Infect Ther. 2012 Nov;10(11):1297-309. doi: 10.1586/eri.12.117. Expert Rev Anti Infect Ther. 2012. PMID: 23241187 Review.
-
A specific interaction of small molecule entry inhibitors with the envelope glycoprotein complex of the Junín hemorrhagic fever arenavirus.J Biol Chem. 2011 Feb 25;286(8):6192-200. doi: 10.1074/jbc.M110.196428. Epub 2010 Dec 15. J Biol Chem. 2011. PMID: 21159779 Free PMC article.
Cited by
-
Arenavirus reverse genetics: new approaches for the investigation of arenavirus biology and development of antiviral strategies.Virology. 2011 Mar 15;411(2):416-25. doi: 10.1016/j.virol.2011.01.013. Epub 2011 Feb 15. Virology. 2011. PMID: 21324503 Free PMC article. Review.
-
Arenavirus Quasispecies and Their Biological Implications.Curr Top Microbiol Immunol. 2016;392:231-76. doi: 10.1007/82_2015_468. Curr Top Microbiol Immunol. 2016. PMID: 26472215 Free PMC article. Review.
-
Distinct Molecular Mechanisms of Host Immune Response Modulation by Arenavirus NP and Z Proteins.Viruses. 2020 Jul 21;12(7):784. doi: 10.3390/v12070784. Viruses. 2020. PMID: 32708250 Free PMC article. Review.
-
Structure-function relationship of the mammarenavirus envelope glycoprotein.Virol Sin. 2016 Oct;31(5):380-394. doi: 10.1007/s12250-016-3815-4. Epub 2016 Aug 4. Virol Sin. 2016. PMID: 27562602 Free PMC article. Review.
-
Highlights in antiviral drug research: antivirals at the horizon.Med Res Rev. 2013 Nov;33(6):1215-48. doi: 10.1002/med.21256. Epub 2012 May 2. Med Res Rev. 2013. PMID: 22553111 Free PMC article. Review.
References
-
- Geisbert, T. W., and Jahrling, P. B. (2004) Nat. Med. 10 S110-121 - PubMed
-
- McCormick, J. B., and Fisher-Hoch, S. P. (2002) Curr. Top Microbiol. Immunol. 262 75-109 - PubMed
-
- Weissenbacher, M. C., Laguens, R. P., and Coto, C. E. (1987) Curr. Top Microbiol. Immunol. 134 79-116 - PubMed
-
- Peters, C. J. (2002) Curr. Top Microbiol. Immunol. 262 65-74 - PubMed
-
- Borio, L., Inglesby, T., Peters, C. J., Schmaljohn, A. L., Hughes, J. M., Jahrling, P. B., Ksiazek, T., Johnson, K. M., Meyerhoff, A., O'Toole, T., Ascher, M. S., Bartlett, J., Breman, J. G., Eitzen, E. M., Jr., Hamburg, M., Hauer, J., Henderson, D. A., Johnson, R. T., Kwik, G., Layton, M., Lillibridge, S., Nabel, G. J., Osterholm, M. T., Perl, T. M., Russell, P., and Tonat, K. (2002) Jama 287 2391-2405 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

