Dicer inactivation leads to progressive functional and structural degeneration of the mouse retina
- PMID: 18463241
- PMCID: PMC3325486
- DOI: 10.1523/JNEUROSCI.0828-08.2008
Dicer inactivation leads to progressive functional and structural degeneration of the mouse retina
Abstract
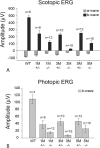
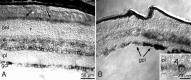
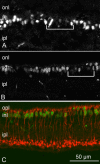
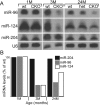
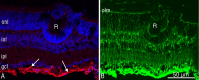
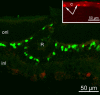
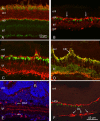
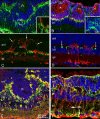
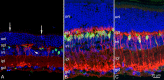
MicroRNAs (miRNAs) are small, highly conserved molecules that have been shown to regulate the expression of genes by binding to specific target mRNAs. Dicer, an RNase III endonuclease, is essential for the production and function of mature miRNAs, and removal of Dicer has been shown to disrupt many developmental processes. In this study, Dicer was removed specifically from the retina using a floxed Dicer conditional allele and the retinal Chx10Cre transgene. Retinal Dicer knock-out mice displayed a reproducible inability to respond to light. In addition, morphological defects were observed with the formation of photoreceptor rosettes at postnatal day 16, which progressed to more general cellular disorganization and widespread degeneration of retinal cell types as the animals aged. This was accompanied by concomitant decrease in both scotopic and photopic electroretinogram (ERG) responses. Interestingly, removing a single allele of Dicer resulted in ERG deficits throughout life but not to morphological abnormalities. Northern blot analysis of Dicer-depleted retinas showed a decrease in several miRNAs. The observation that progressive retinal degeneration occurred after removal of Dicer raises the possibility that miRNAs are involved in retinal neurodegenerative disorders.
Figures

Similar articles
-
Dicer is required for the transition from early to late progenitor state in the developing mouse retina.J Neurosci. 2010 Mar 17;30(11):4048-61. doi: 10.1523/JNEUROSCI.4982-09.2010. J Neurosci. 2010. PMID: 20237275 Free PMC article.
-
Conditional Dicer1 depletion using Chrnb4-Cre leads to cone cell death and impaired photopic vision.Sci Rep. 2019 Feb 19;9(1):2314. doi: 10.1038/s41598-018-38294-9. Sci Rep. 2019. PMID: 30783126 Free PMC article.
-
DICER1 is essential for survival of postmitotic rod photoreceptor cells in mice.FASEB J. 2014 Aug;28(8):3780-91. doi: 10.1096/fj.14-254292. Epub 2014 May 8. FASEB J. 2014. PMID: 24812086 Free PMC article.
-
Dicer cuts the kidney.J Am Soc Nephrol. 2008 Nov;19(11):2043-6. doi: 10.1681/ASN.2008090986. Epub 2008 Oct 15. J Am Soc Nephrol. 2008. PMID: 18923053 Review. No abstract available.
-
Non-coding RNAs in retinal development.Int J Mol Sci. 2012;13(1):558-578. doi: 10.3390/ijms13010558. Epub 2012 Jan 5. Int J Mol Sci. 2012. PMID: 22312272 Free PMC article. Review.
Cited by
-
Restricted perinatal retinal degeneration induces retina reshaping and correlated structural rearrangement of the retinotopic map.Nat Commun. 2013;4:1938. doi: 10.1038/ncomms2926. Nat Commun. 2013. PMID: 23733098 Free PMC article.
-
G9a histone methyltransferase activity in retinal progenitors is essential for proper differentiation and survival of mouse retinal cells.J Neurosci. 2012 Dec 5;32(49):17658-70. doi: 10.1523/JNEUROSCI.1869-12.2012. J Neurosci. 2012. PMID: 23223288 Free PMC article.
-
MicroRNA-215 Regulates Fibroblast Function: Insights from a Human Fibrotic Disease.Cell Cycle. 2015;14(12):1973-84. doi: 10.1080/15384101.2014.998077. Cell Cycle. 2015. PMID: 25565137 Free PMC article.
-
MicroRNAs in the pineal gland: miR-483 regulates melatonin synthesis by targeting arylalkylamine N-acetyltransferase.J Biol Chem. 2012 Jul 20;287(30):25312-24. doi: 10.1074/jbc.M112.356733. J Biol Chem. 2012. PMID: 22908386 Free PMC article.
-
MicroRNAs and neurodegeneration: role and impact.Trends Cell Biol. 2013 Jan;23(1):30-6. doi: 10.1016/j.tcb.2012.08.013. Epub 2012 Sep 28. Trends Cell Biol. 2013. PMID: 23026030 Free PMC article. Review.
References
-
- Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. 2003;113:673–676. - PubMed
-
- Ambros V, Chen X. The regulation of genes and genomes by small RNAs. Development. 2007;134:1635–1641. - PubMed
-
- Barad O, Meiri E, Avniel A, Aharonov R, Barzilai A, Bentwich I, Einav U, Gilad S, Hurban P, Karov Y, Lobenhofer EK, Sharon E, Shiboleth YM, Shtutman M, Bentwich Z, Einat P. MicroRNA expression detected by oligonucleotide microarrays: system establishment and expression profiling in human tissues. Genome Res. 2004;14:2486–2494. - PMC - PubMed
-
- Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R03-DA22201/DA/NIDA NIH HHS/United States
- EY007132/EY/NEI NIH HHS/United States
- R01 EY012654-07/EY/NEI NIH HHS/United States
- R01 EY012654/EY/NEI NIH HHS/United States
- R01 EY009676/EY/NEI NIH HHS/United States
- NS36302/NS/NINDS NIH HHS/United States
- R01 EY012654-08/EY/NEI NIH HHS/United States
- U10 EY013729/EY/NEI NIH HHS/United States
- T32 EY007132/EY/NEI NIH HHS/United States
- P30 EY008571/EY/NEI NIH HHS/United States
- EY008571/EY/NEI NIH HHS/United States
- EY0 9676/EY/NEI NIH HHS/United States
- R01 EY012654-06/EY/NEI NIH HHS/United States
- EY13729/EY/NEI NIH HHS/United States
- EY011123/EY/NEI NIH HHS/United States
- R01-EY12654/EY/NEI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R03 DA022201/DA/NIDA NIH HHS/United States
- P01 NS036302/NS/NINDS NIH HHS/United States
- R01 EY011123/EY/NEI NIH HHS/United States
- R01 EY008064/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
