Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial
- PMID: 18460663
- PMCID: PMC2684623
- DOI: 10.1001/jama.299.17.2027
Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial
Abstract
Context: Recent randomized trials among patients with preexisting cardiovascular disease (CVD) have failed to support benefits of B-vitamin supplementation on cardiovascular risk. Observational data suggest benefits may be greater among women, yet women have been underrepresented in published randomized trials.
Objective: To test whether a combination of folic acid, vitamin B6, and vitamin B12 lowers risk of CVD among high-risk women with and without CVD.
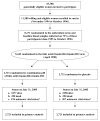
Design, setting, and participants: Within an ongoing randomized trial of antioxidant vitamins, 5442 women who were US health professionals aged 42 years or older, with either a history of CVD or 3 or more coronary risk factors, were enrolled in a randomized, double-blind, placebo-controlled trial to receive a combination pill containing folic acid, vitamin B6, and vitamin B12 or a matching placebo, and were treated for 7.3 years from April 1998 through July 2005.
Intervention: Daily intake of a combination pill of 2.5 mg of folic acid, 50 mg of vitamin B6, and 1 mg of vitamin B12.
Main outcome measures: A composite outcome of myocardial infarction, stroke, coronary revascularization, or CVD mortality.
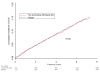
Results: Compared with placebo, a total of 796 women experienced a confirmed CVD event (406 in the active group and 390 in the placebo group). Patients receiving active vitamin treatment had similar risk for the composite CVD primary end point (226.9/10,000 person-years vs 219.2/10,000 person-years for the active vs placebo group; relative risk [RR], 1.03; 95% confidence interval [CI], 0.90-1.19; P = .65), as well as for the secondary outcomes including myocardial infarction (34.5/10,000 person-years vs 39.5/10,000 person-years; RR, 0.87; 95% CI, 0.63-1.22; P = .42), stroke (41.9/10,000 person-years vs 36.8/10,000 person-years; RR, 1.14; 95% CI, 0.82-1.57; P = .44), and CVD mortality (50.3/10,000 person-years vs 49.6/10,000 person-years; RR, 1.01; 95% CI, 0.76-1.35; P = .93). In a blood substudy, geometric mean plasma homocysteine level was decreased by 18.5% (95% CI, 12.5%-24.1%; P < .001) in the active group (n = 150) over that observed in the placebo group (n = 150), for a difference of 2.27 micromol/L (95% CI, 1.54-2.96 micromol/L).
Conclusion: After 7.3 years of treatment and follow-up, a combination pill of folic acid, vitamin B6, and vitamin B12 did not reduce a combined end point of total cardiovascular events among high-risk women, despite significant homocysteine lowering.
Trial registration: clinicaltrials.gov Identifier: NCT00000541.
Figures
Comment in
-
Homocysteine-lowering B vitamin therapy in cardiovascular prevention--wrong again?JAMA. 2008 May 7;299(17):2086-7. doi: 10.1001/jama.299.17.2086. JAMA. 2008. PMID: 18460669 No abstract available.
-
Can folic acid and vitamin B lower CVD risk in high-risk women?J Fam Pract. 2008 Aug;57(8):507-8. J Fam Pract. 2008. PMID: 18697288 No abstract available.
-
Effect of folic acid and B vitamins on cardiovascular disease in women.JAMA. 2008 Sep 24;300(12):1409-10; author reply 1410. doi: 10.1001/jama.300.12.1409-b. JAMA. 2008. PMID: 18812526 No abstract available.
-
Effect of folic acid and B vitamins on cardiovascular disease in women.JAMA. 2008 Sep 24;300(12):1409; author reply 1410. doi: 10.1001/jama.300.12.1409-a. JAMA. 2008. PMID: 18812527 No abstract available.
-
Effect of folic acid and B vitamins on cardiovascular disease in women.JAMA. 2008 Sep 24;300(12):1410; author reply 1410. doi: 10.1001/jama.300.12.1410-a. JAMA. 2008. PMID: 18812529 No abstract available.
Similar articles
-
[Meta-analysis on effect of combined supplementation of folic acid, vitamin B12 and B6 on risk of cardio-cerebrovascular diseases in randomized control trials].Zhonghua Liu Xing Bing Xue Za Zhi. 2016 Jul;37(7):1028-34. doi: 10.3760/cma.j.issn.0254-6450.2016.07.024. Zhonghua Liu Xing Bing Xue Za Zhi. 2016. PMID: 27453118 Chinese.
-
Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomized controlled trial.JAMA. 2008 Aug 20;300(7):795-804. doi: 10.1001/jama.300.7.795. JAMA. 2008. PMID: 18714059 Clinical Trial.
-
Effect of combined folic acid, vitamin B6, and vitamin B12 on cancer risk in women: a randomized trial.JAMA. 2008 Nov 5;300(17):2012-21. doi: 10.1001/jama.2008.555. JAMA. 2008. PMID: 18984888 Free PMC article. Clinical Trial.
-
Homocysteine-lowering interventions for preventing cardiovascular events.Cochrane Database Syst Rev. 2017 Aug 17;8(8):CD006612. doi: 10.1002/14651858.CD006612.pub5. Cochrane Database Syst Rev. 2017. PMID: 28816346 Free PMC article. Review.
-
The effect of long-term homocysteine-lowering on carotid intima-media thickness and flow-mediated vasodilation in stroke patients: a randomized controlled trial and meta-analysis.BMC Cardiovasc Disord. 2008 Sep 20;8:24. doi: 10.1186/1471-2261-8-24. BMC Cardiovasc Disord. 2008. PMID: 18803866 Free PMC article. Review.
Cited by
-
Vitamin B6, Inflammation, and Cardiovascular Outcome in a Population-Based Cohort: The Prevention of Renal and Vascular End-Stage Disease (PREVEND) Study.Nutrients. 2020 Sep 4;12(9):2711. doi: 10.3390/nu12092711. Nutrients. 2020. PMID: 32899820 Free PMC article.
-
Low Vitamin B12 and Lipid Metabolism: Evidence from Pre-Clinical and Clinical Studies.Nutrients. 2020 Jun 29;12(7):1925. doi: 10.3390/nu12071925. Nutrients. 2020. PMID: 32610503 Free PMC article. Review.
-
Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life.Cochrane Database Syst Rev. 2018 Dec 17;12(12):CD011906. doi: 10.1002/14651858.CD011906.pub2. Cochrane Database Syst Rev. 2018. PMID: 30556597 Free PMC article.
-
Plasma total cysteine and total homocysteine and risk of myocardial infarction in women: a prospective study.Am Heart J. 2010 Apr;159(4):599-604. doi: 10.1016/j.ahj.2009.12.037. Am Heart J. 2010. PMID: 20362718 Free PMC article.
-
Association between arsenic exposure from drinking water and proteinuria: results from the Health Effects of Arsenic Longitudinal Study.Int J Epidemiol. 2011 Jun;40(3):828-35. doi: 10.1093/ije/dyr022. Epub 2011 Feb 22. Int J Epidemiol. 2011. PMID: 21343184 Free PMC article.
References
-
- Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288(16):2015–22. - PubMed
-
- Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA. 2006;296(22):2720–6. - PubMed
-
- Carlsson CM. Lowering homocysteine for stroke prevention. Lancet. 2007;369(9576):1841–2. - PubMed
-
- Lonn E, Yusuf S, Arnold MJ, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354(15):1567–77. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

