Disruption of a Plasmodium falciparum cyclic nucleotide phosphodiesterase gene causes aberrant gametogenesis
- PMID: 18452584
- PMCID: PMC2615252
- DOI: 10.1111/j.1365-2958.2008.06267.x
Disruption of a Plasmodium falciparum cyclic nucleotide phosphodiesterase gene causes aberrant gametogenesis
Abstract
Phosphodiesterase (PDE) and guanylyl cyclase (GC) enzymes are key components of the cGMP signalling pathway and are encoded in the genome of Plasmodium falciparum. Here we investigate the role of specific GC and PDE isoforms in gamete formation--a process that is essential for malaria transmission and occurs in the Anopheles mosquito midgut following feeding on an infected individual. Details of the intracellular signalling events controlling development of the male and female gametes from their precursors (gametocytes) remain sparse in P. falciparum. Previous work involving the addition of pharmacological agents to gametocytes implicated cGMP in exflagellation--the emergence of highly motile, flagellated male gametes from the host red blood cell. In this study we show that decreased GC activity in parasites having undergone disruption of the PfGCbeta gene had no significant effect on gametogenesis. By contrast, decreased cGMP-PDE activity during gametocyte development owing to disruption of the PfPDEdelta gene, led to a severely reduced ability to undergo gametogenesis. This suggests that the concentration of cGMP must be maintained below a threshold in the developing gametocyte to allow subsequent differentiation to proceed normally. The data indicate that PfPDEdelta plays a crucial role in regulating cGMP levels during sexual development.
Figures
 and
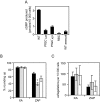
and  ) were assayed for GC activity. Uninfected RBC and WT asexual blood stage particulate fractions were included as controls. The amount of cGMP produced by 104 cells was measured using a 96-well plate cGMP competitive enzyme immunoassay system. The data shown are the mean of five individual assays from multiple parasite cultures carried out in triplicate, with error bars showing the standard error of the mean. B. Rounding up of WT (black bars),
) were assayed for GC activity. Uninfected RBC and WT asexual blood stage particulate fractions were included as controls. The amount of cGMP produced by 104 cells was measured using a 96-well plate cGMP competitive enzyme immunoassay system. The data shown are the mean of five individual assays from multiple parasite cultures carried out in triplicate, with error bars showing the standard error of the mean. B. Rounding up of WT (black bars),  (grey bars) and
(grey bars) and  (white bars) gametocytes was measured after addition of either XA (100 μM) or zaprinast (400 μM). Ten minutes post stimulation, a minimum of 200 live cells were counted by light microscopy and scored as either round or gametocyte-shaped. Results show mean counts for a minimum of four experiments counted in duplicate, with error bars showing the standard error of the mean. The asterisk represents a statistically significant result. C. Exflagellation of WT (black bars),
(white bars) gametocytes was measured after addition of either XA (100 μM) or zaprinast (400 μM). Ten minutes post stimulation, a minimum of 200 live cells were counted by light microscopy and scored as either round or gametocyte-shaped. Results show mean counts for a minimum of four experiments counted in duplicate, with error bars showing the standard error of the mean. The asterisk represents a statistically significant result. C. Exflagellation of WT (black bars),  (grey bars) and
(grey bars) and  (white bars) male gametocytes was measured after addition of either XA (100 μM) or zaprinast (400 μM). Ten minutes post stimulation, cells were observed with a light microscope for 10 min and the number of centres of exflagellation scored per 10 000 cells. Results show mean counts for a minimum of three experiments counted in duplicate, with error bars showing the standard error of the mean.
(white bars) male gametocytes was measured after addition of either XA (100 μM) or zaprinast (400 μM). Ten minutes post stimulation, cells were observed with a light microscope for 10 min and the number of centres of exflagellation scored per 10 000 cells. Results show mean counts for a minimum of three experiments counted in duplicate, with error bars showing the standard error of the mean.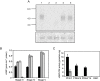
Similar articles
-
Plasmodium falciparum Guanylyl Cyclase-Alpha and the Activity of Its Appended P4-ATPase Domain Are Essential for cGMP Synthesis and Blood-Stage Egress.mBio. 2021 Jan 26;12(1):e02694-20. doi: 10.1128/mBio.02694-20. mBio. 2021. PMID: 33500341 Free PMC article.
-
Plasmodium falciparum Calcium-Dependent Protein Kinase 4 is Critical for Male Gametogenesis and Transmission to the Mosquito Vector.mBio. 2021 Dec 21;12(6):e0257521. doi: 10.1128/mBio.02575-21. Epub 2021 Nov 2. mBio. 2021. PMID: 34724830 Free PMC article.
-
Phosphodiesterase delta governs the mechanical properties of erythrocytes infected with Plasmodium falciparum gametocytes.Microbes Infect. 2023 Jun;25(5):105102. doi: 10.1016/j.micinf.2023.105102. Epub 2023 Jan 25. Microbes Infect. 2023. PMID: 36708871
-
Plasmodium falciparum gametocytes: still many secrets of a hidden life.Mol Microbiol. 2007 Oct;66(2):291-302. doi: 10.1111/j.1365-2958.2007.05904.x. Epub 2007 Sep 3. Mol Microbiol. 2007. PMID: 17784927 Review.
-
cGMP homeostasis in malaria parasites-The key to perceiving and integrating environmental changes during transmission to the mosquito.Mol Microbiol. 2021 May;115(5):829-838. doi: 10.1111/mmi.14633. Epub 2020 Nov 21. Mol Microbiol. 2021. PMID: 33112460 Review.
Cited by
-
cAMP-Signalling Regulates Gametocyte-Infected Erythrocyte Deformability Required for Malaria Parasite Transmission.PLoS Pathog. 2015 May 7;11(5):e1004815. doi: 10.1371/journal.ppat.1004815. eCollection 2015 May. PLoS Pathog. 2015. PMID: 25951195 Free PMC article.
-
Druggable Targets in Cyclic Nucleotide Signaling Pathways in Apicomplexan Parasites and Kinetoplastids against Disabling Protozoan Diseases in Humans.Int J Mol Sci. 2019 Jan 2;20(1):138. doi: 10.3390/ijms20010138. Int J Mol Sci. 2019. PMID: 30609697 Free PMC article. Review.
-
Molecular machinery of signal transduction and cell cycle regulation in Plasmodium.Mol Biochem Parasitol. 2009 May;165(1):1-7. doi: 10.1016/j.molbiopara.2009.01.003. Epub 2009 Jan 21. Mol Biochem Parasitol. 2009. PMID: 19393157 Free PMC article. Review.
-
Phosphoinositide metabolism links cGMP-dependent protein kinase G to essential Ca²⁺ signals at key decision points in the life cycle of malaria parasites.PLoS Biol. 2014 Mar 4;12(3):e1001806. doi: 10.1371/journal.pbio.1001806. eCollection 2014 Mar. PLoS Biol. 2014. PMID: 24594931 Free PMC article.
-
Plasmodium falciparum Guanylyl Cyclase-Alpha and the Activity of Its Appended P4-ATPase Domain Are Essential for cGMP Synthesis and Blood-Stage Egress.mBio. 2021 Jan 26;12(1):e02694-20. doi: 10.1128/mBio.02694-20. mBio. 2021. PMID: 33500341 Free PMC article.
References
-
- Alano P, Billker O. Gametocytes and gametes. In: Sherman IW, editor. Molecular Approaches to Malaria. Washington, DC: American Society for Microbiology Press; 2005. pp. 191–219.
-
- Billker O, Lindo V, Panico M, Etienne AE, Paxton T, Dell A, et al. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature. 1998;392:289–292. - PubMed
-
- Billker O, Shaw MK, Jones IW, Ley SV, Mordue AJ, Sinden RE. Azadirachtin disrupts formation of organised microtubule arrays during microgametogenesis of Plasmodium berghei. J Eukaryot Microbiol. 2002;49:489–497. - PubMed
-
- Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell. 2004;117:503–514. - PubMed
-
- Carucci DJ, Witney AA, Muhia DK, Warhurst DC, Schaap P, Meima M, et al. Guanylyl cyclase activity associated with putative bifunctional integral membrane proteins in Plasmodium falciparum. J Biol Chem. 2000;275:22147–22156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

