Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax)
- PMID: 18451169
- PMCID: PMC4096127
- DOI: 10.1158/0008-5472.CAN-07-1919
Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax)
Abstract
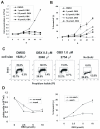
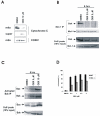
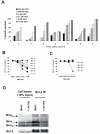
In this study, we investigated the mechanism of apoptosis induction of obatoclax (GX15-070), a novel Bcl-2 homology domain-3 (BH3) mimetic, in acute myeloid leukemia (AML) cell lines and primary AML samples. Obatoclax inhibited cell growth of HL-60, U937, OCI-AML3, and KG-1 cell lines. Apoptosis induction contributed to the observed antiproliferative effects at concentrations of this agent that mirror its affinity for antiapoptotic Bcl-2 proteins. We show that obatoclax can promote the release of cytochrome c from isolated leukemia cell mitochondria and that apoptosis induced by this agent is preceded by the release of Bak from Mcl-1, liberation of Bim from both Bcl-2 and Mcl-1, and the formation of an active Bak/Bax complex. Notably, apoptosis was diminished, but not fully prevented, in the absence of Bak/Bax or Bim, suggesting that obatoclax has additional targets that contribute to its cytotoxicity. At growth inhibitory doses that did not induce apoptosis or decrease viability, obatoclax induced an S-G(2) cell-cycle block. Obatoclax induced apoptosis in AML CD34+ progenitor cells with an average IC(50) of 3.59 +/- 1.23 micromol/L although clonogenicity was inhibited at concentrations of 75 to 100 nmol/L. Obatoclax synergized with the novel BH3 mimetic ABT-737 to induce apoptosis in OCI-AML3 cells and synergistically induced apoptosis in combination with AraC in leukemic cell lines and in primary AML samples. In conclusion, we show that obatoclax potently induces apoptosis and decreases leukemia cell proliferation and may be used in a novel therapeutic strategy for AML alone and in combination with other targeted agents and chemotherapeutics.
Figures

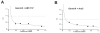
Similar articles
-
Inhibition of Bcl-2 antiapoptotic members by obatoclax potently enhances sorafenib-induced apoptosis in human myeloid leukemia cells through a Bim-dependent process.Blood. 2012 Jun 21;119(25):6089-98. doi: 10.1182/blood-2011-09-378141. Epub 2012 Mar 23. Blood. 2012. PMID: 22446485 Free PMC article.
-
Dual inhibition of Bcl-2 and Bcl-xL strikingly enhances PI3K inhibition-induced apoptosis in human myeloid leukemia cells through a GSK3- and Bim-dependent mechanism.Cancer Res. 2013 Feb 15;73(4):1340-51. doi: 10.1158/0008-5472.CAN-12-1365. Epub 2012 Dec 12. Cancer Res. 2013. PMID: 23243017 Free PMC article.
-
MEK inhibition enhances ABT-737-induced leukemia cell apoptosis via prevention of ERK-activated MCL-1 induction and modulation of MCL-1/BIM complex.Leukemia. 2012 Apr;26(4):778-87. doi: 10.1038/leu.2011.287. Epub 2011 Nov 8. Leukemia. 2012. Retraction in: Leukemia. 2024 Sep;38(9):2072. doi: 10.1038/s41375-024-02339-y PMID: 22064351 Free PMC article. Retracted.
-
BH3 mimetics to improve cancer therapy; mechanisms and examples.Drug Resist Updat. 2007 Dec;10(6):207-17. doi: 10.1016/j.drup.2007.08.002. Epub 2007 Oct 24. Drug Resist Updat. 2007. PMID: 17921043 Free PMC article. Review.
-
Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy.Clin Cancer Res. 2009 Feb 15;15(4):1126-32. doi: 10.1158/1078-0432.CCR-08-0144. Clin Cancer Res. 2009. PMID: 19228717 Free PMC article. Review.
Cited by
-
GX15-070 (obatoclax) induces apoptosis and inhibits cathepsin D- and L-mediated autophagosomal lysis in antiestrogen-resistant breast cancer cells.Mol Cancer Ther. 2013 Apr;12(4):448-59. doi: 10.1158/1535-7163.MCT-12-0617. Epub 2013 Feb 8. Mol Cancer Ther. 2013. PMID: 23395885 Free PMC article.
-
That which does not kill me makes me stronger; combining ERK1/2 pathway inhibitors and BH3 mimetics to kill tumour cells and prevent acquired resistance.Br J Pharmacol. 2013 Aug;169(8):1708-22. doi: 10.1111/bph.12220. Br J Pharmacol. 2013. PMID: 23647573 Free PMC article. Review.
-
Molecular interactions of prodiginines with the BH3 domain of anti-apoptotic Bcl-2 family members.PLoS One. 2013;8(2):e57562. doi: 10.1371/journal.pone.0057562. Epub 2013 Feb 27. PLoS One. 2013. PMID: 23460874 Free PMC article.
-
Manipulating the apoptotic pathway: potential therapeutics for cancer patients.Br J Clin Pharmacol. 2013 Sep;76(3):381-95. doi: 10.1111/bcp.12193. Br J Clin Pharmacol. 2013. PMID: 23782006 Free PMC article. Review.
-
The combination of a histone deacetylase inhibitor with the Bcl-2 homology domain-3 mimetic GX15-070 has synergistic antileukemia activity by activating both apoptosis and autophagy.Clin Cancer Res. 2010 Aug 1;16(15):3923-32. doi: 10.1158/1078-0432.CCR-10-0032. Epub 2010 Jun 10. Clin Cancer Res. 2010. PMID: 20538760 Free PMC article.
References
-
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–12. - PubMed
-
- Fleischer A, Rebollo A, Ayllon V. BH3-only proteins: the lords of death. Arch Immunol Ther Exp (Warsz ) 2003;51:9–17. - PubMed
-
- Willis SN, Fletcher JI, Kaufmann T, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–9. - PubMed
-
- Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. - PubMed
-
- Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10(5):375–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

