Structure of a TrmA-RNA complex: A consensus RNA fold contributes to substrate selectivity and catalysis in m5U methyltransferases
- PMID: 18451029
- PMCID: PMC2383949
- DOI: 10.1073/pnas.0802247105
Structure of a TrmA-RNA complex: A consensus RNA fold contributes to substrate selectivity and catalysis in m5U methyltransferases
Abstract
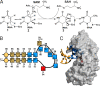
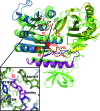
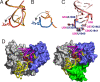
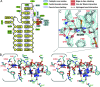
TrmA catalyzes S-adenosylmethionine (AdoMet)-dependent methylation of U54 in most tRNAs. We solved the structure of the Escherichia coli 5-methyluridine (m(5)U) 54 tRNA methyltransferase (MTase) TrmA in a covalent complex with a 19-nt T arm analog to 2.4-A resolution. Mutation of the TrmA catalytic base Glu-358 to Gln arrested catalysis and allowed isolation of the covalent TrmA-RNA complex for crystallization. The protein-RNA interface includes 6 nt of the T loop and two proximal base pairs of the stem. U54 is flipped out of the loop into the active site. A58 occupies the space of the everted U54 and is part of a collinear base stack G53-A58-G57-C56-U55. The RNA fold is different from T loop conformations in unbound tRNA or T arm analogs, but nearly identical to the fold of the RNA loop bound at the active site of the m(5)U MTase RumA. In both enzymes, this consensus fold presents the target U and the following two bases to a conserved binding groove on the protein. Outside of this fold, the RumA and TrmA substrates have completely different structures and protein interfaces. Loop residues other than the target U54 make more than half of their hydrogen bonds to the protein via sugar-phosphate moieties, accounting, in part, for the broad consensus sequence for TrmA substrates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The methyltransferase TrmA facilitates tRNA folding through interaction with its RNA-binding domain.Nucleic Acids Res. 2020 Aug 20;48(14):7981-7990. doi: 10.1093/nar/gkaa548. Nucleic Acids Res. 2020. PMID: 32597953 Free PMC article.
-
Amino acid residues of the Escherichia coli tRNA(m5U54)methyltransferase (TrmA) critical for stability, covalent binding of tRNA and enzymatic activity.Nucleic Acids Res. 2007;35(10):3297-305. doi: 10.1093/nar/gkm205. Epub 2007 Apr 25. Nucleic Acids Res. 2007. PMID: 17459887 Free PMC article.
-
RNA-methyltransferase TrmA is a dual-specific enzyme responsible for C5-methylation of uridine in both tmRNA and tRNA.RNA Biol. 2013 Apr;10(4):572-8. doi: 10.4161/rna.24327. Epub 2013 Apr 1. RNA Biol. 2013. PMID: 23603891 Free PMC article.
-
Substrate recognition by RNA 5-methyluridine methyltransferases and pseudouridine synthases: a structural perspective.J Biol Chem. 2006 Dec 22;281(51):38969-73. doi: 10.1074/jbc.R600034200. Epub 2006 Nov 3. J Biol Chem. 2006. PMID: 17085441 Review. No abstract available.
-
Stereochemical mechanisms of tRNA methyltransferases.FEBS Lett. 2010 Jan 21;584(2):278-86. doi: 10.1016/j.febslet.2009.11.075. FEBS Lett. 2010. PMID: 19944101 Free PMC article. Review.
Cited by
-
Identification and characterization of the Thermus thermophilus 5-methylcytidine (m5C) methyltransferase modifying 23 S ribosomal RNA (rRNA) base C1942.J Biol Chem. 2012 Aug 10;287(33):27593-600. doi: 10.1074/jbc.M112.376160. Epub 2012 Jun 18. J Biol Chem. 2012. PMID: 22711535 Free PMC article.
-
tRNA elbow modifications affect the tRNA pseudouridine synthase TruB and the methyltransferase TrmA.RNA. 2020 Sep;26(9):1131-1142. doi: 10.1261/rna.075473.120. Epub 2020 May 8. RNA. 2020. PMID: 32385137 Free PMC article.
-
Structural insight into the functional mechanism of Nep1/Emg1 N1-specific pseudouridine methyltransferase in ribosome biogenesis.Nucleic Acids Res. 2011 Mar;39(6):2445-57. doi: 10.1093/nar/gkq1131. Epub 2010 Nov 17. Nucleic Acids Res. 2011. PMID: 21087996 Free PMC article.
-
The archaeal COG1901/DUF358 SPOUT-methyltransferase members, together with pseudouridine synthase Pus10, catalyze the formation of 1-methylpseudouridine at position 54 of tRNA.RNA. 2012 Mar;18(3):421-33. doi: 10.1261/rna.030841.111. Epub 2012 Jan 24. RNA. 2012. PMID: 22274953 Free PMC article.
-
The methyltransferase TrmA facilitates tRNA folding through interaction with its RNA-binding domain.Nucleic Acids Res. 2020 Aug 20;48(14):7981-7990. doi: 10.1093/nar/gkaa548. Nucleic Acids Res. 2020. PMID: 32597953 Free PMC article.
References
-
- Moore PB, Steitz TA. The involvement of RNA in ribosome function. Nature. 2002;418:229–235. - PubMed
-
- Ofengand J. Ribosomal RNA pseudouridines and pseudouridine synthases. FEBS Lett. 2002;514:17–25. - PubMed
-
- Kealey JT, Gu X, Santi DV. Enzymatic mechanism of tRNA m5U54 methyltransferase. Biochimie. 1994;76:1133–1142. - PubMed
-
- Agarwalla S, Kealey JT, Santi DV, Stroud RM. Characterization of the 23S ribosomal RNA m5U1939 methyltransferase from Escherichia coli. J Biol Chem. 2002;277:8835–8840. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

