Rapid cloning of high-affinity human monoclonal antibodies against influenza virus
- PMID: 18449194
- PMCID: PMC2515609
- DOI: 10.1038/nature06890
Rapid cloning of high-affinity human monoclonal antibodies against influenza virus
Abstract
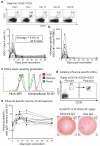
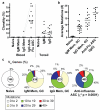
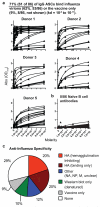
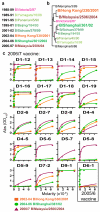
Pre-existing neutralizing antibody provides the first line of defence against pathogens in general. For influenza virus, annual vaccinations are given to maintain protective levels of antibody against the currently circulating strains. Here we report that after booster vaccination there was a rapid and robust influenza-specific IgG+ antibody-secreting plasma cell (ASC) response that peaked at approximately day 7 and accounted for up to 6% of peripheral blood B cells. These ASCs could be distinguished from influenza-specific IgG+ memory B cells that peaked 14-21 days after vaccination and averaged 1% of all B cells. Importantly, as much as 80% of ASCs purified at the peak of the response were influenza specific. This ASC response was characterized by a highly restricted B-cell receptor (BCR) repertoire that in some donors was dominated by only a few B-cell clones. This pauci-clonal response, however, showed extensive intraclonal diversification from accumulated somatic mutations. We used the immunoglobulin variable regions isolated from sorted single ASCs to produce over 50 human monoclonal antibodies (mAbs) that bound to the three influenza vaccine strains with high affinity. This strategy demonstrates that we can generate multiple high-affinity mAbs from humans within a month after vaccination. The panel of influenza-virus-specific human mAbs allowed us to address the issue of original antigenic sin (OAS): the phenomenon where the induced antibody shows higher affinity to a previously encountered influenza virus strain compared with the virus strain present in the vaccine. However, we found that most of the influenza-virus-specific mAbs showed the highest affinity for the current vaccine strain. Thus, OAS does not seem to be a common occurrence in normal, healthy adults receiving influenza vaccination.
Figures
Similar articles
-
Longitudinal analysis of the peripheral B cell repertoire reveals unique effects of immunization with a new influenza virus strain.Genome Med. 2015 Nov 25;7:124. doi: 10.1186/s13073-015-0239-y. Genome Med. 2015. PMID: 26608341 Free PMC article.
-
Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells.Proc Natl Acad Sci U S A. 2012 Jun 5;109(23):9047-52. doi: 10.1073/pnas.1118979109. Epub 2012 May 21. Proc Natl Acad Sci U S A. 2012. PMID: 22615367 Free PMC article.
-
Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells.PLoS One. 2008;3(12):e3942. doi: 10.1371/journal.pone.0003942. Epub 2008 Dec 16. PLoS One. 2008. PMID: 19079604 Free PMC article.
-
Recalling the Future: Immunological Memory Toward Unpredictable Influenza Viruses.Front Immunol. 2019 Jul 2;10:1400. doi: 10.3389/fimmu.2019.01400. eCollection 2019. Front Immunol. 2019. PMID: 31312199 Free PMC article. Review.
-
[Tetravaccine--new fundamental approach to prevention of influenza pandemic].Zh Mikrobiol Epidemiol Immunobiol. 2007 Jul-Aug;(4):15-9. Zh Mikrobiol Epidemiol Immunobiol. 2007. PMID: 17882832 Review. Russian.
Cited by
-
Parainfluenza Virus 5 Priming Followed by SIV/HIV Virus-Like-Particle Boosting Induces Potent and Durable Immune Responses in Nonhuman Primates.Front Immunol. 2021 Feb 25;12:623996. doi: 10.3389/fimmu.2021.623996. eCollection 2021. Front Immunol. 2021. PMID: 33717130 Free PMC article.
-
Characterization of pathogenic human monoclonal autoantibodies against GM-CSF.Proc Natl Acad Sci U S A. 2013 May 7;110(19):7832-7. doi: 10.1073/pnas.1216011110. Epub 2013 Apr 25. Proc Natl Acad Sci U S A. 2013. PMID: 23620516 Free PMC article.
-
Fully human monoclonal antibodies from antibody secreting cells after vaccination with Pneumovax®23 are serotype specific and facilitate opsonophagocytosis.Immunobiology. 2013 May;218(5):745-54. doi: 10.1016/j.imbio.2012.08.278. Epub 2012 Sep 3. Immunobiology. 2013. PMID: 23084371 Free PMC article.
-
Antimalarial antibody repertoire defined by plasma IG proteomics and single B cell IG sequencing.JCI Insight. 2020 Nov 19;5(22):e143471. doi: 10.1172/jci.insight.143471. JCI Insight. 2020. PMID: 33048842 Free PMC article.
-
Circulating Tfh cell and subsets distribution are associated with low-responsiveness to hepatitis B vaccination.Mol Med. 2021 Apr 1;27(1):32. doi: 10.1186/s10020-021-00290-7. Mol Med. 2021. PMID: 33794763 Free PMC article.
References
-
- Francis Thomas., Jr On the Doctrine of Original Antigenic Sin. Proceedings of the American Philosophical Society. 1960;104(6):572–578.
-
- Gerhard W, et al. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol Rev. 1997;159:95–103. - PubMed
-
- Luke Thomas C., Kilbane Edward M., Jackson Jeffrey L., Hoffman Stephen L. Meta-Analysis: Convalescent Blood Products for Spanish Influenza Pneumonia: A Future H5N1 Treatment? Ann Intern Med. 2006;145(8):599–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHSN266200700006C/AI/NIAID NIH HHS/United States
- N01AI50025/AI/NIAID NIH HHS/United States
- U19-AI057266-04/AI/NIAID NIH HHS/United States
- P30 AR053483/AR/NIAMS NIH HHS/United States
- P20 RR018758/RR/NCRR NIH HHS/United States
- R01 AI050933/AI/NIAID NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- N01-AI-50025-02/AI/NIAID NIH HHS/United States
- U19 AI057266-04/AI/NIAID NIH HHS/United States
- P20 RR018758-057588/RR/NCRR NIH HHS/United States
- U19 AI057266/AI/NIAID NIH HHS/United States
- HHSN266200500026C/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

