Search for potential target site of nucleocapsid gene for the design of an epitope-based SARS DNA vaccine
- PMID: 18440652
- PMCID: PMC7112843
- DOI: 10.1016/j.imlet.2008.03.003
Search for potential target site of nucleocapsid gene for the design of an epitope-based SARS DNA vaccine
Abstract
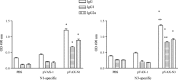
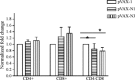
It is believed today that nucleocapsid protein (N) of severe acute respiratory syndrome (SARS)-CoV is one of the most promising antigen candidates for vaccine design. In this study, three fragments [N1 (residues: 1-422); N2 (residues: 1-109); N3 (residues: 110-422)] of N protein of SARS-CoV were expressed in Escherichia coli and analyzed by pooled sera of convalescence phase of SARS patients. Three gene fragments [N1 (1-1269 nt), N2 (1-327 nt) and N3 (328-1269 nt)-expressing the same proteins of N1, N2 and N3, respectively] of SARS-N were cloned into pVAX-1 and used to immunize BALB/c mice by electroporation. Humoral (by enzyme-linked immunosorbent assay, ELISA) and cellular (by cell proliferation and CD4(+):CD8(+) assay) immunity was detected by using recombinant N1 and N3 specific antigen. Results showed that N1 and N3 fragments of N protein expressed by E. coli were able to react with sera of SARS patients but N2 could not. Specific humoral and cellular immunity in mice could be induced significantly by inoculating SARS-CoV N1 and N3 DNA vaccine. In addition, the immune response levels in N3 were significantly higher for antibody responses (IgG and IgG1 but not IgG2a) and cell proliferation but not in CD4(+):CD8(+) assay compared to N1 vaccine. The identification of antigenic N protein fragments has implications to provide basic information for the design of DNA vaccine against SARS-CoV. The present results not only suggest that DNA immunization with pVax-N3 could be used as potential DNA vaccination approaches to induce antibody in BALB/c mice, but also illustrates that gene immunization with these SARS DNA vaccines can generate different immune responses.
Figures



Similar articles
-
Priming with SARS CoV S DNA and boosting with SARS CoV S epitopes specific for CD4+ and CD8+ T cells promote cellular immune responses.Vaccine. 2007 Sep 28;25(39-40):6981-91. doi: 10.1016/j.vaccine.2007.06.047. Epub 2007 Jul 16. Vaccine. 2007. PMID: 17709158 Free PMC article.
-
Immunological characterizations of the nucleocapsid protein based SARS vaccine candidates.Vaccine. 2006 Apr 12;24(16):3100-8. doi: 10.1016/j.vaccine.2006.01.058. Epub 2006 Feb 8. Vaccine. 2006. PMID: 16494977 Free PMC article.
-
Induction of specific immune responses by severe acute respiratory syndrome coronavirus spike DNA vaccine with or without interleukin-2 immunization using different vaccination routes in mice.Clin Vaccine Immunol. 2007 Jul;14(7):894-901. doi: 10.1128/CVI.00019-07. Epub 2007 May 9. Clin Vaccine Immunol. 2007. PMID: 17494640 Free PMC article.
-
Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus.Avian Pathol. 2003 Dec;32(6):567-82. doi: 10.1080/03079450310001621198. Avian Pathol. 2003. PMID: 14676007 Free PMC article. Review.
-
Obstacles and advances in SARS vaccine development.Vaccine. 2006 Feb 13;24(7):863-71. doi: 10.1016/j.vaccine.2005.08.102. Epub 2005 Sep 12. Vaccine. 2006. PMID: 16191455 Free PMC article. Review.
Cited by
-
Reverse genetic systems: Rational design of coronavirus live attenuated vaccines with immune sequelae.Adv Virus Res. 2020;107:383-416. doi: 10.1016/bs.aivir.2020.06.003. Epub 2020 Jun 30. Adv Virus Res. 2020. PMID: 32711735 Free PMC article. Review.
-
Vaccine development against coronavirus (2003 to present): An overview, recent advances, current scenario, opportunities and challenges.Diabetes Metab Syndr. 2020 Sep-Oct;14(5):1361-1376. doi: 10.1016/j.dsx.2020.07.022. Epub 2020 Jul 21. Diabetes Metab Syndr. 2020. PMID: 32755836 Free PMC article. Review.
-
Epitope-Based Vaccine Target Screening against Highly Pathogenic MERS-CoV: An In Silico Approach Applied to Emerging Infectious Diseases.PLoS One. 2015 Dec 7;10(12):e0144475. doi: 10.1371/journal.pone.0144475. eCollection 2015. PLoS One. 2015. PMID: 26641892 Free PMC article.
-
Dimerization of SARS-CoV-2 nucleocapsid protein affects sensitivity of ELISA based diagnostics of COVID-19.Int J Biol Macromol. 2022 Mar 1;200:428-437. doi: 10.1016/j.ijbiomac.2022.01.094. Epub 2022 Jan 17. Int J Biol Macromol. 2022. PMID: 35051498 Free PMC article.
-
The spike protein of SARS-CoV--a target for vaccine and therapeutic development.Nat Rev Microbiol. 2009 Mar;7(3):226-36. doi: 10.1038/nrmicro2090. Epub 2009 Feb 9. Nat Rev Microbiol. 2009. PMID: 19198616 Free PMC article. Review.
References
-
- Drosten C., Gunther S., Preiser W., Werf S.V.D., Brodt H.R., Becker S. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. New Engl J Med. 2003;348:967–976. - PubMed
-
- Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. - PubMed
-
- Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. - PubMed
-
- Holmes K.V., Enjuanes L. The SARS coronavirus: a postgenomic era. Science. 2003;300:1377–1378. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

