Insulin-like growth factor-I stimulates Shc-dependent phosphatidylinositol 3-kinase activation via Grb2-associated p85 in vascular smooth muscle cells
- PMID: 18420583
- PMCID: PMC2423238
- DOI: 10.1074/jbc.M801687200
Insulin-like growth factor-I stimulates Shc-dependent phosphatidylinositol 3-kinase activation via Grb2-associated p85 in vascular smooth muscle cells
Abstract
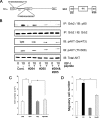
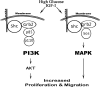
Insulin-like growth factor-I (IGF-I) stimulates vascular smooth muscle cell proliferation and migration by activating both MAPK and phosphatidylinositol 3-kinase (PI3K). Vascular smooth muscle cells (VSMCs) maintained in 25 mm glucose sustain MAPK activation via increased Shc phosphorylation and Grb2 association resulting in an enhanced mitogenic response compared with cells grown in 5 mm glucose. PI3K plays a major role in IGF-I-stimulated VSMC migration, and hyperglycemia augments this response. In contrast to MAPK activation the role of Shc in modulating PI3K in response to IGF-I has not been determined. In this study we show that impaired Shc association with Grb2 results in decreased Grb2-p85 association, SHPS-1-p85 recruitment, and PI3K activation in response to IGF-I. Exposure of VSMCs to cell-permeable peptides, which contained polyproline sequences from p85 proposed to mediate Grb2 association, resulted in inhibition of Grb2-p85 binding and AKT phosphorylation. Transfected cells that expressed p85 mutant that had specific prolines mutated to alanines resulted in less Grb2-p85 association, and a Grb2 mutant (W36A/W193A) that attenuated p85 binding showed decreased association of p85 with SHPS-1, PI3K activation, AKT phosphorylation, cell proliferation, and migration in response to IGF-I. Cellular exposure to 25 mm glucose, which is required for Shc phosphorylation in response to IGF-I, resulted in enhanced Grb2 binding to p85, activation of PI3K activity, and increased AKT phosphorylation as compared with cells exposed to 5 mm glucose. We conclude that in VSMCs exposed to hyperglycemia, IGF-I stimulation of Shc facilitates the transfer of Grb2 to p85 resulting in enhanced PI3K activation and AKT phosphorylation leading to enhanced cell proliferation and migration.
Figures




Similar articles
-
Role of SHPS-1 in the regulation of insulin-like growth factor I-stimulated Shc and mitogen-activated protein kinase activation in vascular smooth muscle cells.Mol Biol Cell. 2005 Jul;16(7):3353-64. doi: 10.1091/mbc.e04-10-0918. Epub 2005 May 11. Mol Biol Cell. 2005. PMID: 15888547 Free PMC article.
-
p66shc negatively regulates insulin-like growth factor I signal transduction via inhibition of p52shc binding to Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 leading to impaired growth factor receptor-bound protein-2 membrane recruitment.Mol Endocrinol. 2008 Sep;22(9):2162-75. doi: 10.1210/me.2008-0079. Epub 2008 Jul 7. Mol Endocrinol. 2008. PMID: 18606861 Free PMC article.
-
Hyperglycemia-induced p66shc inhibits insulin-like growth factor I-dependent cell survival via impairment of Src kinase-mediated phosphoinositide-3 kinase/AKT activation in vascular smooth muscle cells.Endocrinology. 2010 Aug;151(8):3611-23. doi: 10.1210/en.2010-0242. Epub 2010 Jun 9. Endocrinology. 2010. PMID: 20534722 Free PMC article.
-
The IGF-1 Signaling Pathway in Viral Infections.Viruses. 2021 Jul 29;13(8):1488. doi: 10.3390/v13081488. Viruses. 2021. PMID: 34452353 Free PMC article. Review.
-
Igf-I signaling in response to hyperglycemia and the development of diabetic complications.Curr Diabetes Rev. 2011 Jul;7(4):235-45. doi: 10.2174/157339911796397848. Curr Diabetes Rev. 2011. PMID: 21707534 Review.
Cited by
-
Signal regulatory protein alpha (SIRPalpha)/CD47 interaction and function.Curr Opin Immunol. 2009 Feb;21(1):47-52. doi: 10.1016/j.coi.2009.01.008. Epub 2009 Feb 14. Curr Opin Immunol. 2009. PMID: 19223164 Free PMC article. Review.
-
Age-related changes in redox signaling and VSMC function.Antioxid Redox Signal. 2010 Mar 1;12(5):641-55. doi: 10.1089/ars.2009.2854. Antioxid Redox Signal. 2010. PMID: 19737090 Free PMC article. Review.
-
The Strategies for Treating "Alzheimer's Disease": Insulin Signaling May Be a Feasible Target.Curr Issues Mol Biol. 2022 Dec 7;44(12):6172-6188. doi: 10.3390/cimb44120421. Curr Issues Mol Biol. 2022. PMID: 36547082 Free PMC article. Review.
-
Evolving mechanisms of vascular smooth muscle contraction highlight key targets in vascular disease.Biochem Pharmacol. 2018 Jul;153:91-122. doi: 10.1016/j.bcp.2018.02.012. Epub 2018 Feb 13. Biochem Pharmacol. 2018. PMID: 29452094 Free PMC article. Review.
-
IGF-I stimulates cooperative interaction between the IGF-I receptor and CSK homologous kinase that regulates SHPS-1 phosphorylation in vascular smooth muscle cells.Mol Endocrinol. 2011 Sep;25(9):1636-49. doi: 10.1210/me.2011-0035. Epub 2011 Jul 28. Mol Endocrinol. 2011. PMID: 21799000 Free PMC article.
References
-
- Clemmons, D. R. (2007) Nat. Rev. Drug Discov. 6 821–833 - PubMed
-
- Ling, Y., Maile, L. A., and Clemmons, D. R. (2003) Mol. Endocrinol. 17 1824–1833 - PubMed
-
- Imai, Y., and Clemmons, D. R. (1999) Endocrinology 140 4228–4235 - PubMed
-
- Maile, L. A., Capps, B. E., Ling, Y., Xi, G., and Clemmons, D. R. (2007) Endocrinology 148 2435–2443 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

