Sex differences in brain developing in the presence or absence of gonads
- PMID: 18418875
- PMCID: PMC2575820
- DOI: 10.1002/dneu.20638
Sex differences in brain developing in the presence or absence of gonads
Abstract
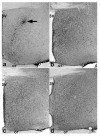
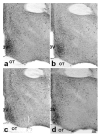
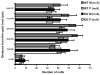
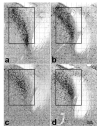
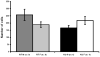
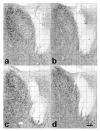
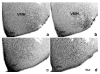
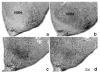
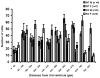
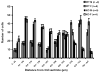
Brain sexual differentiation results from the interaction of genetic and hormonal influences. This study used a unique agonadal mouse model to determine relative contributions of genetic and gonadal hormone influences in the differentiation of selected brain regions. SF-1 knockout (SF-1 KO) mice are born without gonads and adrenal glands and are not exposed to endogenous sex steroids during fetal/neonatal development. Consequently, male and female SF-1 KO mice are born with female external genitalia and if left on their own, die shortly after birth due to adrenal insufficiency. In this study, SF-1 KO mice were rescued by neonatal adrenal transplantation to examine their brain morphology in adult life. To determine potential brain loci that might mediate functional sex differences, we examined the area and distribution of immunoreactive calbindin and neuronal nitric oxide synthase in the preoptic area (POA) and ventromedial nucleus of the hypothalamus, two areas previously reported to be sexually dimorphic in the mammalian brain. A sex difference in the positioning of cells containing immunoreactive calbindin in a group within the POA was clearly gonad dependent based on the elimination of the sex difference in SF-1 KO mice. Several other differences in the area of ventromedial hypothalamus and in POA were maintained in male and female SF-1 KO mice, suggesting gonad-independent genetic influences on sexually dimorphic brain development.
Figures

Similar articles
-
The Influence of Gonadal Steroid Hormones on Immunoreactive Kisspeptin in the Preoptic Area and Arcuate Nucleus of Developing Agonadal Mice with a Genetic Disruption of Steroidogenic Factor 1.Neuroendocrinology. 2016;103(3-4):248-58. doi: 10.1159/000437166. Epub 2015 Jun 30. Neuroendocrinology. 2016. PMID: 26138474 Free PMC article.
-
Neuronal nitric oxide synthase and calbindin delineate sex differences in the developing hypothalamus and preoptic area.Dev Neurobiol. 2007 Sep 1;67(10):1371-81. doi: 10.1002/dneu.20507. Dev Neurobiol. 2007. PMID: 17638388 Free PMC article.
-
Aggressive behaviors in adult SF-1 knockout mice that are not exposed to gonadal steroids during development.Behav Neurosci. 2008 Aug;122(4):876-84. doi: 10.1037/0735-7044.122.4.876. Behav Neurosci. 2008. PMID: 18729641 Free PMC article.
-
The roles of steroidogenic factor 1 in endocrine development and function.Mol Cell Endocrinol. 1998 Oct 25;145(1-2):15-20. doi: 10.1016/s0303-7207(98)00164-6. Mol Cell Endocrinol. 1998. PMID: 9922094 Review.
-
SF-1: a key regulator of development and function in the mammalian reproductive system.Acta Paediatr Jpn. 1996 Aug;38(4):412-9. doi: 10.1111/j.1442-200x.1996.tb03516.x. Acta Paediatr Jpn. 1996. PMID: 8840555 Review.
Cited by
-
Hormonal programming across the lifespan.Horm Metab Res. 2012 Jul;44(8):577-86. doi: 10.1055/s-0032-1312593. Epub 2012 Jun 14. Horm Metab Res. 2012. PMID: 22700441 Free PMC article. Review.
-
Brain sex differences and hormone influences: a moving experience?J Neuroendocrinol. 2009 Mar;21(4):387-92. doi: 10.1111/j.1365-2826.2009.01834.x. J Neuroendocrinol. 2009. PMID: 19207813 Free PMC article. Review.
-
A primer on the use of mouse models for identifying direct sex chromosome effects that cause sex differences in non-gonadal tissues.Biol Sex Differ. 2016 Dec 13;7:68. doi: 10.1186/s13293-016-0115-5. eCollection 2016. Biol Sex Differ. 2016. PMID: 27999654 Free PMC article. Review.
-
The Influence of Gonadal Steroid Hormones on Immunoreactive Kisspeptin in the Preoptic Area and Arcuate Nucleus of Developing Agonadal Mice with a Genetic Disruption of Steroidogenic Factor 1.Neuroendocrinology. 2016;103(3-4):248-58. doi: 10.1159/000437166. Epub 2015 Jun 30. Neuroendocrinology. 2016. PMID: 26138474 Free PMC article.
-
The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues.Horm Behav. 2009 May;55(5):570-8. doi: 10.1016/j.yhbeh.2009.03.011. Horm Behav. 2009. PMID: 19446073 Free PMC article. Review.
References
-
- Arnold AP. Sex chromosomes and brain gender. Nat Rev Neurosci. 2004;5:701–708. - PubMed
-
- Beatty WW, O’Briant DA, Vilberg TR. Effects of ovariectomy and estradiol injections on food intake and body weight in rats with ventromedial hypothalamic lesions. Pharmacol Biochem Behav. 1975;3:539–544. - PubMed
-
- Bell DD, Zucker I. Sex differences in body weight and eating: organization and activation by gonadal hormones in the rat. Physiol Behav. 1971;7:27–34. - PubMed
-
- Bland ML, Fowkes RC, Ingraham HA. Differential requirement for steroidogenic factor-1 gene dosage in adrenal development versus endocrine function. Mol Endocrinol. 2004;18:941–52. - PubMed
-
- Brager DH, Sickel MJ, McCarthy MM. Developmental sex differences in calbindin-D(28K) and calretinin immunoreactivity in the neonatal rat hypothalamus. J Neurobiol. 2000;42:315–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

