Tight junctions and the modulation of barrier function in disease
- PMID: 18415116
- PMCID: PMC2413111
- DOI: 10.1007/s00418-008-0424-9
Tight junctions and the modulation of barrier function in disease
Abstract
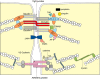
Tight junctions create a paracellular barrier in epithelial and endothelial cells protecting them from the external environment. Two different classes of integral membrane proteins constitute the tight junction strands in epithelial cells and endothelial cells, occludin and members of the claudin protein family. In addition, cytoplasmic scaffolding molecules associated with these junctions regulate diverse physiological processes like proliferation, cell polarity and regulated diffusion. In many diseases, disruption of this regulated barrier occurs. This review will briefly describe the molecular composition of the tight junctions and then present evidence of the link between tight junction dysfunction and disease.
Figures
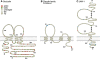
Similar articles
-
Tight junctions in neurological diseases.Acta Neurobiol Exp (Wars). 2011;71(4):393-408. doi: 10.55782/ane-2011-1861. Acta Neurobiol Exp (Wars). 2011. PMID: 22237490 Review.
-
The tight junction: a multifunctional complex.Am J Physiol Cell Physiol. 2004 Jun;286(6):C1213-28. doi: 10.1152/ajpcell.00558.2003. Am J Physiol Cell Physiol. 2004. PMID: 15151915 Review.
-
Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein.J Cell Biol. 1996 Aug;134(4):1031-49. doi: 10.1083/jcb.134.4.1031. J Cell Biol. 1996. PMID: 8769425 Free PMC article.
-
Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function.J Cell Sci. 2003 Feb 15;116(Pt 4):725-42. doi: 10.1242/jcs.00300. J Cell Sci. 2003. PMID: 12538773
-
Possible involvement of tight junctions, extracellular matrix and nuclear receptors in epithelial differentiation.J Biomed Biotechnol. 2011;2011:253048. doi: 10.1155/2011/253048. Epub 2011 Nov 17. J Biomed Biotechnol. 2011. PMID: 22162632 Free PMC article. Review.
Cited by
-
Fluid-Based Protein Biomarkers in Traumatic Brain Injury: The View from the Bedside.Int J Mol Sci. 2023 Nov 13;24(22):16267. doi: 10.3390/ijms242216267. Int J Mol Sci. 2023. PMID: 38003454 Free PMC article. Review.
-
The zinc sensing receptor, ZnR/GPR39, triggers metabotropic calcium signalling in colonocytes and regulates occludin recovery in experimental colitis.Philos Trans R Soc Lond B Biol Sci. 2016 Aug 5;371(1700):20150420. doi: 10.1098/rstb.2015.0420. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27377730 Free PMC article.
-
The functional antagonist of sphingosine-1-phosphate, FTY720, impairs gut barrier function.Front Pharmacol. 2024 Aug 19;15:1407228. doi: 10.3389/fphar.2024.1407228. eCollection 2024. Front Pharmacol. 2024. PMID: 39224783 Free PMC article.
-
Teneligliptin protects against ischemia/reperfusion-induced endothelial permeability in vivo and in vitro.RSC Adv. 2020 Jan 22;10(7):3765-3774. doi: 10.1039/c9ra08810e. eCollection 2020 Jan 22. RSC Adv. 2020. PMID: 35492650 Free PMC article.
-
A tight junction-associated Merlin-angiomotin complex mediates Merlin's regulation of mitogenic signaling and tumor suppressive functions.Cancer Cell. 2011 Apr 12;19(4):527-40. doi: 10.1016/j.ccr.2011.02.017. Cancer Cell. 2011. PMID: 21481793 Free PMC article.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '16371949', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16371949/'}]}
- Abbott NJ, Ronnback L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Review. Nat Rev Neurosci 7:41–53 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PMC', 'value': 'PMC2115479', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC2115479/'}, {'type': 'PubMed', 'value': '2568363', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/2568363/'}]}
- Achler C, Filmer D, Merte C, Drenckhahn D (1989) Role of microtubules in polarized delivery of apical membrane proteins to the brush border of the intestinal epithelium. J Cell Biol 109:179–189 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '11698262', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11698262/'}]}
- Ahdieh M, Vandenbos T, Youakim A (2001) Lung epithelial barrier function and wound healing are decreased by IL-4 and IL-13 and enhanced by IFN-gamma. Am J Physiol Cell Physiol 281:C2029–C2038 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PMC', 'value': 'PMC2120780', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC2120780/'}, {'type': 'PubMed', 'value': '8601611', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/8601611/'}]}
- Ando-Akatsuka Y, Saitou M, Hirase T, Kishi M, Sakakibara A, Itoh M, Yonemura S, Furuse M, Tsukita S (1996) Interspecies diversity of the occludin sequence: cDNA cloning of human, mouse, dog, and rat-kangaroo homologues. J Cell Biol 133:43–47 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '9836530', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9836530/'}]}
- Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW (1998) Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes 47:1953–1959 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

