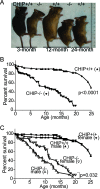
CHIP deficiency decreases longevity, with accelerated aging phenotypes accompanied by altered protein quality control
- PMID: 18411298
- PMCID: PMC2423116
- DOI: 10.1128/MCB.00296-08
CHIP deficiency decreases longevity, with accelerated aging phenotypes accompanied by altered protein quality control
Abstract
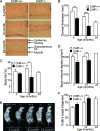
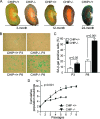
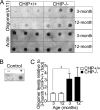
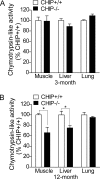
During the course of biological aging, there is a gradual accumulation of damaged proteins and a concomitant functional decline in the protein degradation system. Protein quality control is normally ensured by the coordinated actions of molecular chaperones and the protein degradation system that collectively help to maintain protein homeostasis. The carboxyl terminus of Hsp70-interacting protein (CHIP), a ubiquitin ligase/cochaperone, participates in protein quality control by targeting a broad range of chaperone substrates for proteasome degradation via the ubiquitin-proteasome system, demonstrating a broad involvement of CHIP in maintaining cytoplasmic protein quality control. In the present study, we have investigated the influence that protein quality control exerts on the aging process by using CHIP-/- mice. CHIP deficiency in mice leads to a markedly reduced life span, along with accelerated age-related pathophysiological phenotypes. These features were accompanied by indications of accelerated cellular senescence and increased indices of oxidative stress. In addition, CHIP-/- mice exhibit a deregulation of protein quality control, as indicated by elevated levels of toxic oligomer proteins and a decline in proteasome activity. Taken together, these data reveal that impaired protein quality control contributes to cellular senescence and implicates CHIP-dependent quality control mechanisms in the regulation of mammalian longevity in vivo.
Figures
Similar articles
-
Regulation of the cytoplasmic quality control protein degradation pathway by BAG2.J Biol Chem. 2005 Nov 18;280(46):38673-81. doi: 10.1074/jbc.M507986200. Epub 2005 Sep 16. J Biol Chem. 2005. PMID: 16169850
-
CHIP E3 ligase regulates mammalian senescence by modulating the levels of oxidized proteins.Mech Ageing Dev. 2011 May;132(5):269-72. doi: 10.1016/j.mad.2011.04.003. Epub 2011 Apr 12. Mech Ageing Dev. 2011. PMID: 21510971
-
Cooperation of a ubiquitin domain protein and an E3 ubiquitin ligase during chaperone/proteasome coupling.Curr Biol. 2001 Oct 16;11(20):1569-77. doi: 10.1016/s0960-9822(01)00487-0. Curr Biol. 2001. PMID: 11676916
-
CHIP: a co-chaperone for degradation by the proteasome.Subcell Biochem. 2015;78:219-42. doi: 10.1007/978-3-319-11731-7_11. Subcell Biochem. 2015. PMID: 25487024 Review.
-
CHIP: a quality-control E3 ligase collaborating with molecular chaperones.Int J Biochem Cell Biol. 2003 May;35(5):572-8. doi: 10.1016/s1357-2725(02)00394-1. Int J Biochem Cell Biol. 2003. PMID: 12672450 Review.
Cited by
-
Chaperone-assisted E3 ligase CHIP: A double agent in cancer.Genes Dis. 2021 Sep 1;9(6):1521-1555. doi: 10.1016/j.gendis.2021.08.003. eCollection 2022 Nov. Genes Dis. 2021. PMID: 36157498 Free PMC article. Review.
-
CHIP has a protective role against oxidative stress-induced cell death through specific regulation of endonuclease G.Cell Death Dis. 2013 Jun 13;4(6):e666. doi: 10.1038/cddis.2013.181. Cell Death Dis. 2013. PMID: 23764847 Free PMC article.
-
Facilitating Akt clearance via manipulation of Hsp70 activity and levels.J Biol Chem. 2010 Jan 22;285(4):2498-505. doi: 10.1074/jbc.M109.057208. Epub 2009 Nov 4. J Biol Chem. 2010. PMID: 19889640 Free PMC article.
-
CHIP Haploinsufficiency Exacerbates Hepatic Steatosis via Enhanced TXNIP Expression and Endoplasmic Reticulum Stress Responses.Antioxidants (Basel). 2023 Feb 11;12(2):458. doi: 10.3390/antiox12020458. Antioxidants (Basel). 2023. PMID: 36830016 Free PMC article.
-
Ubiquitin Ligases in Longevity and Aging Skeletal Muscle.Int J Mol Sci. 2022 Jul 9;23(14):7602. doi: 10.3390/ijms23147602. Int J Mol Sci. 2022. PMID: 35886949 Free PMC article. Review.
References
-
- Ballinger, C. A., P. Connell, Y. Wu, Z. Hu, L. J. Thompson, L.-Y. Yin, and C. Patterson. 1999. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol. Cell. Biol. 194535-4545. - PMC - PubMed
-
- Barral, J. M., S. A. Broadley, G. Schaffar, and F. U. Hartl. 2004. Roles of molecular chaperones in protein misfolding diseases. Semin. Cell Dev. Biol. 1517-29. - PubMed
-
- Bence, N. F., R. M. Sampat, and R. R. Kopito. 2001. Impairment of the ubiquitin-proteasome system by protein aggregation. Science 2921552-1555. - PubMed
-
- Bokov, A., A. Chaudhuri, and A. Richardson. 2004. The role of oxidative damage and stress in aging. Mech. Ageing Dev. 125811-826. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
