An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models
- PMID: 18403709
- PMCID: PMC4322935
- DOI: 10.1126/science.1154986
An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models
Abstract
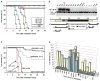
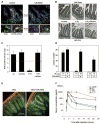
The toxicity of ionizing radiation is associated with massive apoptosis in radiosensitive organs. Here, we investigate whether a drug that activates a signaling mechanism used by tumor cells to suppress apoptosis can protect healthy cells from the harmful effects of radiation. We studied CBLB502, a polypeptide drug derived from Salmonella flagellin that binds to Toll-like receptor 5 (TLR5) and activates nuclear factor-kappaB signaling. A single injection of CBLB502 before lethal total-body irradiation protected mice from both gastrointestinal and hematopoietic acute radiation syndromes and resulted in improved survival. CBLB502 injected after irradiation also enhanced survival, but at lower radiation doses. It is noteworthy that the drug did not decrease tumor radiosensitivity in mouse models. CBLB502 also showed radioprotective activity in lethally irradiated rhesus monkeys. Thus, TLR5 agonists could potentially improve the therapeutic index of cancer radiotherapy and serve as biological protectants in radiation emergencies.
Figures


Comment in
-
Medicine. Drug bestows radiation resistance on mice and monkeys.Science. 2008 Apr 11;320(5873):163. doi: 10.1126/science.320.5873.163. Science. 2008. PMID: 18403680 No abstract available.
Similar articles
-
Radioprotective effect of newly synthesized toll-like receptor 5 agonist, KMRC011, in mice exposed to total-body irradiation.J Radiat Res. 2019 Jul 1;60(4):432-441. doi: 10.1093/jrr/rrz024. J Radiat Res. 2019. PMID: 31165150 Free PMC article.
-
Medicine. Drug bestows radiation resistance on mice and monkeys.Science. 2008 Apr 11;320(5873):163. doi: 10.1126/science.320.5873.163. Science. 2008. PMID: 18403680 No abstract available.
-
Central role of liver in anticancer and radioprotective activities of Toll-like receptor 5 agonist.Proc Natl Acad Sci U S A. 2013 May 14;110(20):E1857-66. doi: 10.1073/pnas.1222805110. Epub 2013 Apr 29. Proc Natl Acad Sci U S A. 2013. PMID: 23630282 Free PMC article.
-
[Biologic modulation of ionizing radiation by Toll-like receptors agonists: towards an increase in the therapeutic index of radiotherapy?].Bull Cancer. 2012 May;99(5):545-50. doi: 10.1684/bdc.2012.1578. Bull Cancer. 2012. PMID: 22522695 Review. French.
-
Entolimod as a radiation countermeasure for acute radiation syndrome.Drug Discov Today. 2021 Jan;26(1):17-30. doi: 10.1016/j.drudis.2020.10.003. Epub 2020 Oct 13. Drug Discov Today. 2021. PMID: 33065293 Review.
Cited by
-
Novel drugs to ameliorate gastrointestinal normal tissue radiation toxicity in clinical practice: what is emerging from the laboratory?Curr Opin Support Palliat Care. 2012 Mar;6(1):54-9. doi: 10.1097/SPC.0b013e32834e3bd7. Curr Opin Support Palliat Care. 2012. PMID: 22228028 Free PMC article. Review.
-
Small molecule modulators of immune pattern recognition receptors.RSC Chem Biol. 2023 Oct 23;4(12):1014-1036. doi: 10.1039/d3cb00096f. eCollection 2023 Nov 29. RSC Chem Biol. 2023. PMID: 38033733 Free PMC article. Review.
-
Disruption of Notch signaling aggravates irradiation-induced bone marrow injury, which is ameliorated by a soluble Dll1 ligand through Csf2rb2 upregulation.Sci Rep. 2016 May 18;6:26003. doi: 10.1038/srep26003. Sci Rep. 2016. PMID: 27188577 Free PMC article.
-
Synergistic effect of the TLR5 agonist CBLB502 and its downstream effector IL-22 against liver injury.Cell Death Dis. 2021 Apr 6;12(4):366. doi: 10.1038/s41419-021-03654-3. Cell Death Dis. 2021. PMID: 33824326 Free PMC article.
-
Medical Countermeasures for Radiation Exposure and Related Injuries: Characterization of Medicines, FDA-Approval Status and Inclusion into the Strategic National Stockpile.Health Phys. 2015 Jun;108(6):607-30. doi: 10.1097/HP.0000000000000279. Health Phys. 2015. PMID: 25905522 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

