Hearing loss alters the subcellular distribution of presynaptic GAD and postsynaptic GABAA receptors in the auditory cortex
- PMID: 18403398
- PMCID: PMC2583158
- DOI: 10.1093/cercor/bhn044
Hearing loss alters the subcellular distribution of presynaptic GAD and postsynaptic GABAA receptors in the auditory cortex
Abstract
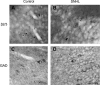
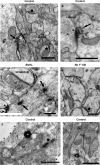
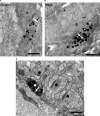
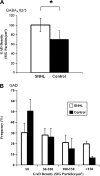
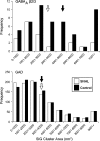
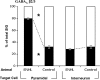
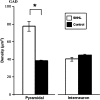
We have shown previously that auditory experience regulates the maturation of excitatory synapses in the auditory cortex (ACx). In this study, we used electron microscopic immunocytochemistry to determine whether the heightened excitability of the ACx following neonatal sensorineural hearing loss (SNHL) also involves pre- or postsynaptic alterations of GABAergic synapses. SNHL was induced in gerbils just prior to the onset of hearing (postnatal day 10). At P17, the gamma-aminobutyri acid type A (GABA(A)) receptor's beta2/3-subunit (GABA(A)beta2/3) clusters residing at plasma membranes in layers 2/3 of ACx was reduced significantly in size (P < 0.05) and number (P < 0.005), whereas the overall number of immunoreactive puncta (intracellular + plasmalemmal) remained unchanged. The reduction of GABA(A)beta2/3 was observed along perikaryal plasma membranes of excitatory neurons but not of GABAergic interneurons. This cell-specific change can contribute to the enhanced excitability of SNHL ACx. Presynaptically, GABAergic axon terminals were significantly larger but less numerous and contained 47% greater density of glutamic acid decarboxylase immunoreactivity (P < 0.05). This suggests that GABA synthesis may be upregulated by a retrograde signal arising from lowered levels of postsynaptic GABA(A)R. Thus, both, the pre- and postsynaptic sides of inhibitory synapses that form upon pyramidal neurons of the ACx are regulated by neonatal auditory experience.
Figures

Similar articles
-
Hearing loss prevents the maturation of GABAergic transmission in the auditory cortex.Cereb Cortex. 2008 Sep;18(9):2098-108. doi: 10.1093/cercor/bhm233. Epub 2008 Jan 24. Cereb Cortex. 2008. PMID: 18222937 Free PMC article.
-
Spontaneous activity in the developing gerbil auditory cortex in vivo involves GABAergic transmission.Neuroscience. 2012 Dec 13;226:130-44. doi: 10.1016/j.neuroscience.2012.09.008. Epub 2012 Sep 15. Neuroscience. 2012. PMID: 22986170 Free PMC article.
-
Hearing loss raises excitability in the auditory cortex.J Neurosci. 2005 Apr 13;25(15):3908-18. doi: 10.1523/JNEUROSCI.5169-04.2005. J Neurosci. 2005. PMID: 15829643 Free PMC article.
-
GABA distribution in a pain-modulating zone of trigeminal subnucleus interpolaris.Somatosens Res. 1988;5(3):205-17. doi: 10.3109/07367228809144627. Somatosens Res. 1988. PMID: 2895952 Review.
-
Developmental plasticity of auditory cortical inhibitory synapses.Hear Res. 2011 Sep;279(1-2):140-8. doi: 10.1016/j.heares.2011.03.015. Epub 2011 Apr 2. Hear Res. 2011. PMID: 21463668 Free PMC article. Review.
Cited by
-
The cytoplasmic localization of ADNP through 14-3-3 promotes sex-dependent neuronal morphogenesis, cortical connectivity, and calcium signaling.Mol Psychiatry. 2023 May;28(5):1946-1959. doi: 10.1038/s41380-022-01939-3. Epub 2023 Jan 11. Mol Psychiatry. 2023. PMID: 36631597
-
Somatosensory and visual crossmodal plasticity in the anterior auditory field of early-deaf cats.Hear Res. 2011 Oct;280(1-2):38-47. doi: 10.1016/j.heares.2011.02.004. Epub 2011 Feb 24. Hear Res. 2011. PMID: 21354286 Free PMC article.
-
Auditory map plasticity: diversity in causes and consequences.Curr Opin Neurobiol. 2014 Feb;24(1):143-56. doi: 10.1016/j.conb.2013.11.009. Epub 2013 Dec 13. Curr Opin Neurobiol. 2014. PMID: 24492090 Free PMC article. Review.
-
Fast-spiking GABA circuit dynamics in the auditory cortex predict recovery of sensory processing following peripheral nerve damage.Elife. 2017 Mar 21;6:e21452. doi: 10.7554/eLife.21452. Elife. 2017. PMID: 28323619 Free PMC article.
-
Regulation of auditory plasticity during critical periods and following hearing loss.Hear Res. 2020 Nov;397:107976. doi: 10.1016/j.heares.2020.107976. Epub 2020 Apr 20. Hear Res. 2020. PMID: 32591097 Free PMC article. Review.
References
-
- Abbott SD, Hughes LF, Bauer CA, Salvi R, Caspary DM. Detection of Glutamate Decarboxylase isoforms in rat inferior colliculus following acoustic exposure. Neuroscience. 1999;93:1375–1381. - PubMed
-
- Amin J, Weiss DS. GABAA receptor needs two homologous domains of the beta-subunit for activation by GABA but not by pentobarbital. Nature. 1993;366:565–569. - PubMed
-
- Barnes EM., Jr Use-dependent regulation of GABAA receptors. Int Rev Neurobiol. 1996;39:53–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS041091/NS/NINDS NIH HHS/United States
- P30 EY013079/EY/NEI NIH HHS/United States
- R01 EY013145/EY/NEI NIH HHS/United States
- EY13145/EY/NEI NIH HHS/United States
- R25 NS080686/NS/NINDS NIH HHS/United States
- P30 EY13079/EY/NEI NIH HHS/United States
- DC006864/DC/NIDCD NIH HHS/United States
- NS41091/NS/NINDS NIH HHS/United States
- R01 DA00961809/DA/NIDA NIH HHS/United States
- R01 EY013145-04/EY/NEI NIH HHS/United States
- P30 EY013079-109003/EY/NEI NIH HHS/United States
- R01 NS041091-04/NS/NINDS NIH HHS/United States

