Activation of p300 histone acetyltransferase activity is an early endothelial response to laminar shear stress and is essential for stimulation of endothelial nitric-oxide synthase mRNA transcription
- PMID: 18397880
- PMCID: PMC2423243
- DOI: 10.1074/jbc.M801803200
Activation of p300 histone acetyltransferase activity is an early endothelial response to laminar shear stress and is essential for stimulation of endothelial nitric-oxide synthase mRNA transcription
Abstract
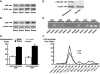
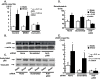
Previous studies have shown that the acute stimulation of endothelial nitric-oxide synthase (eNOS) mRNA transcription by laminar shear stress is dependent on nuclear factor kappa B (NFkappaB) subunits p50 and p65 binding to a shear stress response element (SSRE) in the human eNOS promoter and that mutation of the SSRE abrogates the shear-stimulated increase in eNOS promoter activity. In the present study, we found that although shear markedly increased eNOS mRNA, the increase in nuclear translocation of p50 and p65 caused by shear was only 2-fold, suggesting that shear has additional effects on NFkappaB cofactor activity beyond nuclear translocation. Chromatin immunoprecipitation assays showed that virtually no p50 or p65 was bound to the eNOS promoter at base line but that shear increased the binding of these subunits to the human eNOS SSRE by 10- to 20-fold. Co-immunoprecipitation studies demonstrated during the first 30 min of shear p300 bound to p65. Shear also increased p300 histone acetyltransferase (HAT) activity by 2.5-fold and increased acetylation of p65. The increase in eNOS mRNA caused by shear was completely blocked by pharmacological inhibition of p300/HAT activity with curcumin or by p300 small interfering RNA. Chromatin immunoprecipitation assays also showed that shear stimulated acetylation of histones 3 and 4 at the region of the eNOS promoter SSRE and extended 3' toward the eNOS coding region. This was associated with opening of chromatin at the SSRE. In conclusion, these studies reveal a previously unknown role of p300/HAT activation as a very early response to shear that is essential for increasing eNOS mRNA levels.
Figures

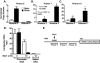
Similar articles
-
A negative feedback mechanism involving nitric oxide and nuclear factor kappa-B modulates endothelial nitric oxide synthase transcription.J Mol Cell Cardiol. 2005 Oct;39(4):595-603. doi: 10.1016/j.yjmcc.2005.06.012. J Mol Cell Cardiol. 2005. PMID: 16099468
-
Induction of KLF2 by fluid shear stress requires a novel promoter element activated by a phosphatidylinositol 3-kinase-dependent chromatin-remodeling pathway.J Biol Chem. 2005 Jun 17;280(24):23371-9. doi: 10.1074/jbc.M413839200. Epub 2005 Apr 15. J Biol Chem. 2005. PMID: 15834135
-
Shear stress regulates endothelial nitric-oxide synthase promoter activity through nuclear factor kappaB binding.J Biol Chem. 2004 Jan 2;279(1):163-8. doi: 10.1074/jbc.M307528200. Epub 2003 Oct 21. J Biol Chem. 2004. PMID: 14570928
-
Endothelial nitric oxide synthase: a new paradigm for gene regulation in the injured blood vessel.Arterioscler Thromb Vasc Biol. 2004 Mar;24(3):405-12. doi: 10.1161/01.ATV.0000109171.50229.33. Epub 2003 Dec 1. Arterioscler Thromb Vasc Biol. 2004. PMID: 14656742 Review.
-
Acetyltransferase p300 Is a Putative Epidrug Target for Amelioration of Cellular Aging-Related Cardiovascular Disease.Cells. 2021 Oct 22;10(11):2839. doi: 10.3390/cells10112839. Cells. 2021. PMID: 34831061 Free PMC article. Review.
Cited by
-
Thioredoxin interacting protein promotes endothelial cell inflammation in response to disturbed flow by increasing leukocyte adhesion and repressing Kruppel-like factor 2.Circ Res. 2012 Feb 17;110(4):560-8. doi: 10.1161/CIRCRESAHA.111.256362. Epub 2012 Jan 19. Circ Res. 2012. PMID: 22267843 Free PMC article.
-
DNA Methyltransferase 1-Dependent DNA Hypermethylation Constrains Arteriogenesis by Augmenting Shear Stress Set Point.J Am Heart Assoc. 2017 Nov 30;6(12):e007673. doi: 10.1161/JAHA.117.007673. J Am Heart Assoc. 2017. PMID: 29191807 Free PMC article.
-
Histone Modifications and Their Contributions to Hypertension.Hypertension. 2024 Feb;81(2):229-239. doi: 10.1161/HYPERTENSIONAHA.123.21755. Epub 2023 Nov 30. Hypertension. 2024. PMID: 38031837 Review.
-
Vascular mechanotransduction.Physiol Rev. 2023 Apr 1;103(2):1247-1421. doi: 10.1152/physrev.00053.2021. Epub 2023 Jan 5. Physiol Rev. 2023. PMID: 36603156 Free PMC article. Review.
-
Epigenetics in cardiovascular disease.Curr Opin Cardiol. 2011 May;26(3):209-15. doi: 10.1097/HCO.0b013e328345986e. Curr Opin Cardiol. 2011. PMID: 21415727 Free PMC article. Review.
References
-
- James, N. L., Harrison, D. G., and Nerem, R. M. (1995) FASEB J. 9 968–973 - PubMed
-
- Berk, B. C., Corson, M. A., Peterson, T. E., and Tseng, H. (1995) J. Biomech. 28 1439–1450 - PubMed
-
- Malek, A. M., and Izumo, S. (1995) J. Biomech. 28 1515–1528 - PubMed
-
- Nerem, R. M., Harrison, D. G., Taylor, W. R., and Alexander, R. W. (1993) J. Cardiovasc. Pharmacol. 21 Suppl. 1, S6–S10 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

