Interleukin-10-induced MARCH1 mediates intracellular sequestration of MHC class II in monocytes
- PMID: 18389477
- PMCID: PMC2759377
- DOI: 10.1002/eji.200737902
Interleukin-10-induced MARCH1 mediates intracellular sequestration of MHC class II in monocytes
Abstract
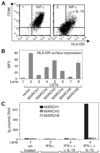
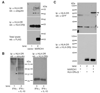
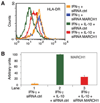
IL-10 is a potent anti-inflammatory cytokine interfering with antigen presentation by inducing the intracellular sequestration of MHC class II (MHC-II) molecules. Here we studied the contribution of membrane-associated RING-CH (MARCH) ubiquitin ligase family members to the IL-10-induced down-regulation of MHC-II molecules. We found that MARCH1 and MARCH8 proteins are the most potent family members for the down-regulation of MHC-II surface expression in transfected cells, but only MARCH1 mRNA expression is strongly induced by IL-10 in human primary monocytes. We detected mono- and poly-ubiquitinated forms of MHC-II molecules both in IL-10-treated monocytes and in cells transfected with MARCH1. We also show direct interaction between MHC-II and MARCH1 molecules in co-immunoprecipitation assays. Finally, we found that siRNA-mediated knockdown of MARCH1 reverses IL-10-induced MHC-II down-regulation in primary monocytes. Thus, the immunosuppressive effect of IL-10 on antigen presentation is mediated through induced expression of MARCH1.
Conflict of interest statement
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Figures
Similar articles
-
MARCH1 down-regulation in IL-10-activated B cells increases MHC class II expression.Cytokine. 2012 Jul;59(1):27-30. doi: 10.1016/j.cyto.2012.03.015. Epub 2012 Apr 12. Cytokine. 2012. PMID: 22503116
-
Tollip-induced down-regulation of MARCH1.Results Immunol. 2013 Feb 20;3:17-25. doi: 10.1016/j.rinim.2013.02.002. eCollection 2013. Results Immunol. 2013. PMID: 24600555 Free PMC article.
-
Autoregulation of MARCH1 expression by dimerization and autoubiquitination.J Immunol. 2012 May 15;188(10):4959-70. doi: 10.4049/jimmunol.1102708. Epub 2012 Apr 16. J Immunol. 2012. PMID: 22508929
-
MARCH ligases in immunity.Curr Opin Immunol. 2019 Jun;58:38-43. doi: 10.1016/j.coi.2019.03.001. Epub 2019 May 4. Curr Opin Immunol. 2019. PMID: 31063934 Review.
-
Inhibition of the MHC class II antigen presentation pathway by human cytomegalovirus.Curr Top Microbiol Immunol. 2002;269:101-15. doi: 10.1007/978-3-642-59421-2_7. Curr Top Microbiol Immunol. 2002. PMID: 12224504 Review.
Cited by
-
Targeting the MHC Class II antigen presentation pathway in cancer immunotherapy.Oncoimmunology. 2012 Sep 1;1(6):908-916. doi: 10.4161/onci.21205. Oncoimmunology. 2012. PMID: 23162758 Free PMC article.
-
Interleukin-10 Family and Tuberculosis: An Old Story Renewed.Int J Biol Sci. 2016 Apr 27;12(6):710-7. doi: 10.7150/ijbs.13881. eCollection 2016. Int J Biol Sci. 2016. PMID: 27194948 Free PMC article. Review.
-
Present Yourself! By MHC Class I and MHC Class II Molecules.Trends Immunol. 2016 Nov;37(11):724-737. doi: 10.1016/j.it.2016.08.010. Epub 2016 Sep 7. Trends Immunol. 2016. PMID: 27614798 Free PMC article. Review.
-
Differential down-regulation of HLA-DR on monocyte subpopulations during systemic inflammation.Crit Care. 2010;14(2):R61. doi: 10.1186/cc8959. Epub 2010 Apr 13. Crit Care. 2010. PMID: 20385017 Free PMC article.
-
March1-dependent modulation of donor MHC II on CD103+ dendritic cells mitigates alloimmunity.Nat Commun. 2018 Aug 28;9(1):3482. doi: 10.1038/s41467-018-05572-z. Nat Commun. 2018. PMID: 30154416 Free PMC article.
References
-
- Steimle V, Otten LA, Zufferey M, Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome) Cell. 1993;75:135–146. - PubMed
-
- LeibundGut-Landmann S, Waldburger JM, Krawczyk M, Otten LA, Suter T, Fontana A, Acha-Orbea H, Reith W. Mini-review: Specificity and expression of CIITA, the master regulator of MHC class II genes. Eur. J. Immunol. 2004;34:1513–1525. - PubMed
-
- Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. - PubMed
-
- Pierre P, Turley SJ, Gatti E, Hull M, Meltzer J, Mirza A, Inaba K, et al. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

