Characterization and site-directed mutagenesis of Wzb, an O-phosphatase from Lactobacillus rhamnosus
- PMID: 18387182
- PMCID: PMC2364625
- DOI: 10.1186/1471-2091-9-10
Characterization and site-directed mutagenesis of Wzb, an O-phosphatase from Lactobacillus rhamnosus
Abstract
Background: Reversible phosphorylation events within a polymerisation complex have been proposed to modulate capsular polysaccharide synthesis in Streptococcus pneumoniae. Similar phosphatase and kinase genes are present in the exopolysaccharide (EPS) biosynthesis loci of numerous lactic acid bacteria genomes.
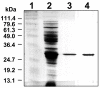
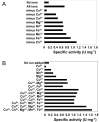
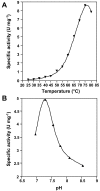
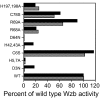
Results: The protein sequence deduced from the wzb gene in Lactobacillus rhamnosus ATCC 9595 reveals four motifs of the polymerase and histidinol phosphatase (PHP) superfamily of prokaryotic O-phosphatases. Native and modified His-tag fusion Wzb proteins were purified from Escherichia coli cultures. Extracts showed phosphatase activity towards tyrosine-containing peptides. The purified fusion protein Wzb was active on p-nitrophenyl-phosphate (pNPP), with an optimal activity in presence of bovine serum albumin (BSA 1%) at pH 7.3 and a temperature of 75 degrees C. At 50 degrees C, residual activity decreased to 10 %. Copper ions were essential for phosphatase activity, which was significantly increased by addition of cobalt. Mutated fusion Wzb proteins exhibited reduced phosphatase activity on p-nitrophenyl-phosphate. However, one variant (C6S) showed close to 20% increase in phosphatase activity.
Conclusion: These characteristics reveal significant differences with the manganese-dependent CpsB protein tyrosine phosphatase described for Streptococcus pneumoniae as well as with the polysaccharide-related phosphatases of Gram negative bacteria.
Figures

Similar articles
-
Crystal structures of Wzb of Escherichia coli and CpsB of Streptococcus pneumoniae, representatives of two families of tyrosine phosphatases that regulate capsule assembly.J Mol Biol. 2009 Sep 25;392(3):678-88. doi: 10.1016/j.jmb.2009.07.026. Epub 2009 Jul 16. J Mol Biol. 2009. PMID: 19616007 Free PMC article.
-
Sequence-based identification of inositol monophosphatase-like histidinol-phosphate phosphatases (HisN) in Corynebacterium glutamicum, Actinobacteria, and beyond.BMC Microbiol. 2017 Jul 18;17(1):161. doi: 10.1186/s12866-017-1069-4. BMC Microbiol. 2017. PMID: 28720084 Free PMC article.
-
Structural and mechanistic characterization of L-histidinol phosphate phosphatase from the polymerase and histidinol phosphatase family of proteins.Biochemistry. 2013 Feb 12;52(6):1101-12. doi: 10.1021/bi301496p. Epub 2013 Jan 30. Biochemistry. 2013. PMID: 23327428 Free PMC article.
-
Manganese-dependent protein O-phosphatases in prokaryotes and their biological functions.Front Biosci. 2004 May 1;9:1382-97. doi: 10.2741/1318. Front Biosci. 2004. PMID: 14977554 Review.
-
Structure, Mechanism, and Substrate Profiles of the Trinuclear Metallophosphatases from the Amidohydrolase Superfamily.Methods Enzymol. 2018;607:187-216. doi: 10.1016/bs.mie.2018.04.019. Epub 2018 Jul 19. Methods Enzymol. 2018. PMID: 30149858 Review.
Cited by
-
Crystal structures of Wzb of Escherichia coli and CpsB of Streptococcus pneumoniae, representatives of two families of tyrosine phosphatases that regulate capsule assembly.J Mol Biol. 2009 Sep 25;392(3):678-88. doi: 10.1016/j.jmb.2009.07.026. Epub 2009 Jul 16. J Mol Biol. 2009. PMID: 19616007 Free PMC article.
-
Capsular polysaccharide inhibits adhesion of Bifidobacterium longum 105-A to enterocyte-like Caco-2 cells and phagocytosis by macrophages.Gut Pathog. 2017 May 1;9:27. doi: 10.1186/s13099-017-0177-x. eCollection 2017. Gut Pathog. 2017. PMID: 28469711 Free PMC article.
-
Cellular Mn/Zn Ratio Influences Phosphoglucomutase Activity and Capsule Production in Streptococcus pneumoniae D39.J Bacteriol. 2021 Jun 8;203(13):e0060220. doi: 10.1128/JB.00602-20. Epub 2021 Jun 8. J Bacteriol. 2021. PMID: 33875543 Free PMC article.
-
Streptococcus pneumoniae phosphotyrosine phosphatase CpsB and alterations in capsule production resulting from changes in oxygen availability.J Bacteriol. 2014 Jun;196(11):1992-2003. doi: 10.1128/JB.01545-14. Epub 2014 Mar 21. J Bacteriol. 2014. PMID: 24659769 Free PMC article.
-
Revisiting histidine-dependent acid phosphatases: a distinct group of tyrosine phosphatases.Trends Biochem Sci. 2009 Jun;34(6):273-8. doi: 10.1016/j.tibs.2009.03.002. Epub 2009 May 19. Trends Biochem Sci. 2009. PMID: 19467874 Free PMC article.
References
-
- Morona JK, Morona R, Miller DC, Paton JC. Mutational analysis of the carboxy-terminal (YGX)4 repeat domain of CpsD, an autophosphorylating tyrosine kinase required for capsule biosynthesis in Streptococcus pneumoniae. J Bacteriol. 2003;185:3009–3019. doi: 10.1128/JB.185.10.3009-3019.2003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

