Regulation of proline-rich Akt substrate of 40 kDa (PRAS40) function by mammalian target of rapamycin complex 1 (mTORC1)-mediated phosphorylation
- PMID: 18372248
- PMCID: PMC2414301
- DOI: 10.1074/jbc.M800723200
Regulation of proline-rich Akt substrate of 40 kDa (PRAS40) function by mammalian target of rapamycin complex 1 (mTORC1)-mediated phosphorylation
Abstract
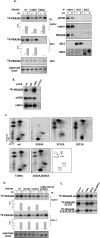
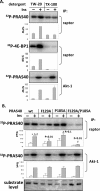
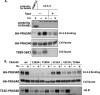
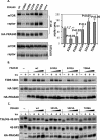
The rapamycin-sensitive mammalian target of rapamycin (mTOR) complex 1 (mTORC1) contains mTOR, raptor, mLST8, and PRAS40 (proline-rich Akt substrate of 40 kDa). PRAS40 functions as a negative regulator when bound to mTORC1, and it dissociates from mTORC1 in response to insulin. PRAS40 has been demonstrated to be a substrate of mTORC1, and one phosphorylation site, Ser-183, has been identified. In this study, we used two-dimensional phosphopeptide mapping in conjunction with mutational analysis to show that in addition to Ser-183, mTORC1 also phosphorylates Ser-212 and Ser-221 in PRAS40 when assayed in vitro. Mutation of all three residues to Ala markedly reduces mTORC1-mediated phosphorylation of PRAS40 in vitro. All three sites were confirmed to be phosphorylated in vivo by [(32)P]orthophosphate labeling and peptide mapping. Phosphorylation of Ser-221 and Ser-183 but not Ser-212 is sensitive to rapamycin treatment. Furthermore, we demonstrate that mutation of Ser-221 to Ala reduces the interaction with 14-3-3 to the same extent as mutation of Thr-246, the Akt/protein kinase B-phosphorylated site. We also find that mutation of Ser-221 to Ala increases the inhibitory activity of PRAS40 toward mTORC1. We propose that after mTORC1 kinase activation by upstream regulators, PRAS40 is phosphorylated directly by mTOR, thus contributing to the relief of PRAS40-mediated substrate competition.
Figures

Similar articles
-
The proline-rich Akt substrate of 40 kDa (PRAS40) is a physiological substrate of mammalian target of rapamycin complex 1.J Biol Chem. 2007 Jul 13;282(28):20329-39. doi: 10.1074/jbc.M702636200. Epub 2007 May 21. J Biol Chem. 2007. PMID: 17517883 Free PMC article.
-
PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding.J Biol Chem. 2007 Jul 6;282(27):20036-44. doi: 10.1074/jbc.M702376200. Epub 2007 May 17. J Biol Chem. 2007. PMID: 17510057
-
Alcohol and PRAS40 knockdown decrease mTOR activity and protein synthesis via AMPK signaling and changes in mTORC1 interaction.J Cell Biochem. 2010 Apr 15;109(6):1172-84. doi: 10.1002/jcb.22496. J Cell Biochem. 2010. PMID: 20127721 Free PMC article.
-
PRAS40: target or modulator of mTORC1 signalling and insulin action?Arch Physiol Biochem. 2009 Oct;115(4):163-75. doi: 10.1080/13813450902988580. Arch Physiol Biochem. 2009. PMID: 19480563 Review.
-
Proline-rich Akt substrate of 40kDa (PRAS40): a novel downstream target of PI3k/Akt signaling pathway.Cell Signal. 2012 Jan;24(1):17-24. doi: 10.1016/j.cellsig.2011.08.010. Epub 2011 Aug 31. Cell Signal. 2012. PMID: 21906675 Review.
Cited by
-
Intestinal cell kinase (ICK) promotes activation of mTOR complex 1 (mTORC1) through phosphorylation of Raptor Thr-908.J Biol Chem. 2012 Apr 6;287(15):12510-9. doi: 10.1074/jbc.M111.302117. Epub 2012 Feb 22. J Biol Chem. 2012. PMID: 22356909 Free PMC article.
-
Regulation of blood-testis barrier (BTB) dynamics during spermatogenesis via the "Yin" and "Yang" effects of mammalian target of rapamycin complex 1 (mTORC1) and mTORC2.Int Rev Cell Mol Biol. 2013;301:291-358. doi: 10.1016/B978-0-12-407704-1.00006-3. Int Rev Cell Mol Biol. 2013. PMID: 23317821 Free PMC article. Review.
-
Uropathogenic Escherichia coli Infection Compromises the Blood-Testis Barrier by Disturbing mTORC1-mTORC2 Balance.Front Immunol. 2021 Feb 19;12:582858. doi: 10.3389/fimmu.2021.582858. eCollection 2021. Front Immunol. 2021. PMID: 33679734 Free PMC article.
-
Regulation of mTOR complex 1 (mTORC1) by raptor Ser863 and multisite phosphorylation.J Biol Chem. 2010 Jan 1;285(1):80-94. doi: 10.1074/jbc.M109.029637. Epub 2009 Oct 28. J Biol Chem. 2010. PMID: 19864431 Free PMC article.
-
mTor signaling in skeletal muscle during sepsis and inflammation: where does it all go wrong?Physiology (Bethesda). 2011 Apr;26(2):83-96. doi: 10.1152/physiol.00044.2010. Physiology (Bethesda). 2011. PMID: 21487027 Free PMC article. Review.
References
-
- Wullschleger, S., Loewith, R., and Hall, M. N. (2006) Cell 124 471–484 - PubMed
-
- Yang, Q., and Guan, K. L. (2007) Cell Res. 17 666–681 - PubMed
-
- Brunn, G. J., Fadden, P., Haystead, T. A., and Lawrence, J. C., Jr. (1997) J. Biol. Chem. 272 32547–32550 - PubMed
-
- Schalm, S. S., and Blenis, J. (2002) Curr. Biol. 12 632–639 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

