Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP)
- PMID: 18364357
- PMCID: PMC2386911
- DOI: 10.1074/jbc.M802139200
Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP)
Abstract
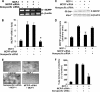
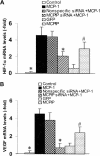
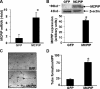
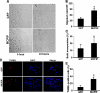
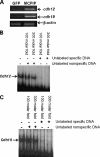
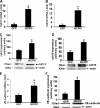
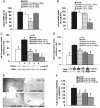
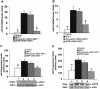
Monocyte chemotactic protein-1 (MCP-1) has been recognized as an angiogenic chemokine. The molecular mechanism of MCP-1-mediated angiogenesis remains unknown. We recently identified a novel transcription factor, designated MCP-1-induced protein (MCPIP), in human monocytes after treatment with MCP-1. We investigated whether MCP-1-induced angiogenesis is mediated via MCPIP. Treatment of human umbilical vein endothelial cells (HUVECs) with MCP-1 induced expression of MCPIP and capillary-like tube formation. Knockdown of MCPIP by small interfering RNA (siRNA) suppressed MCP-1-induced angiogenesis-related gene VEGF and HIF-1alpha expression as well as tube formation. Transfection of HUVECs with an MCPIP expression vector induced angiogenesis-related genes and tube formation. Chromatin immunoprecipitation analysis revealed that cadherin (cdh) 12 and cdh19 are in vivo targets of MCPIP. Transfection of HUVECs with MCPIP expression vector activated the expression of cdh12 and cdh19 genes. Knockdown of cdh12 or cdh19 expression markedly inhibited MCPIP-induced capillary-like tube formation. Moreover, knockdown of MCPIP also significantly suppressed MCP-1-induced cdh12 and cdh19 gene expression. Our data strongly suggest that MCP-1-induced angiogenesis is mediated via MCPIP, at least in part through transcriptional activation of cdh12 and cdh19.
Figures
Similar articles
-
Monocyte chemotactic protein-induced protein (MCPIP) promotes inflammatory angiogenesis via sequential induction of oxidative stress, endoplasmic reticulum stress and autophagy.Cell Signal. 2012 Nov;24(11):2123-31. doi: 10.1016/j.cellsig.2012.07.014. Epub 2012 Jul 20. Cell Signal. 2012. PMID: 22820500
-
MCP-1-induced protein promotes endothelial-like and angiogenic properties in human bone marrow monocytic cells.J Pharmacol Exp Ther. 2013 Nov;347(2):288-97. doi: 10.1124/jpet.113.207316. Epub 2013 Sep 5. J Pharmacol Exp Ther. 2013. PMID: 24008336 Free PMC article.
-
Antidicer RNAse activity of monocyte chemotactic protein-induced protein-1 is critical for inducing angiogenesis.Am J Physiol Cell Physiol. 2013 Nov 15;305(10):C1021-32. doi: 10.1152/ajpcell.00203.2013. Epub 2013 Sep 18. Am J Physiol Cell Physiol. 2013. PMID: 24048733 Free PMC article.
-
Role of MCP-1 in cardiovascular disease: molecular mechanisms and clinical implications.Clin Sci (Lond). 2009 Jul 2;117(3):95-109. doi: 10.1042/CS20080581. Clin Sci (Lond). 2009. PMID: 19566488 Review.
-
Monocyte Chemotactic Protein-Induced Protein 1 (MCPIP-1): A Key Player of Host Defense and Immune Regulation.Front Immunol. 2021 Oct 1;12:727861. doi: 10.3389/fimmu.2021.727861. eCollection 2021. Front Immunol. 2021. PMID: 34659213 Free PMC article. Review.
Cited by
-
MCP-1 (monocyte chemotactic protein-1)-induced protein, a recently identified zinc finger protein, induces adipogenesis in 3T3-L1 pre-adipocytes without peroxisome proliferator-activated receptor gamma.J Biol Chem. 2009 Oct 2;284(40):27620-8. doi: 10.1074/jbc.M109.025320. Epub 2009 Aug 7. J Biol Chem. 2009. PMID: 19666473 Free PMC article.
-
Keratinocyte-specific ablation of Mcpip1 impairs skin integrity and promotes local and systemic inflammation.J Mol Med (Berl). 2019 Dec;97(12):1669-1684. doi: 10.1007/s00109-019-01853-2. Epub 2019 Nov 30. J Mol Med (Berl). 2019. PMID: 31786670 Free PMC article.
-
The Immune-Centric Revolution in the Diabetic Foot: Monocytes and Lymphocytes Role in Wound Healing and Tissue Regeneration-A Narrative Review.J Clin Med. 2022 Feb 8;11(3):889. doi: 10.3390/jcm11030889. J Clin Med. 2022. PMID: 35160339 Free PMC article. Review.
-
Targeting Intramembrane Protein-Protein Interactions: Novel Therapeutic Strategy of Millions Years Old.Adv Protein Chem Struct Biol. 2018;111:61-99. doi: 10.1016/bs.apcsb.2017.06.004. Epub 2017 Jul 24. Adv Protein Chem Struct Biol. 2018. PMID: 29459036 Free PMC article. Review.
-
Autophagy: controlling cell fate in rheumatic diseases.Nat Rev Rheumatol. 2016 Sep;12(9):517-31. doi: 10.1038/nrrheum.2016.92. Epub 2016 Jun 23. Nat Rev Rheumatol. 2016. PMID: 27334205 Review.
References
-
- Griffioen, A. W., and Molema, G. (2000) Pharmacol. Rev. 52 237-268 - PubMed
-
- Pandya, N. M., Dhalla, N. S., and Santani, D. D. (2006) Vasc. Pharmacol. 44 265-274 - PubMed
-
- Herold, J., Pipp, F., Fernandez, B., Xing, Z., Heil, M., Tillmanns, H., and Braun-Dullaeus, R. C. (2004) Hum. Gene Ther. 15 1-12 - PubMed
-
- Charo, I. F., and Taubman, M. B. (2004) Circ. Res. 95 858-866 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

